Upregulation of miR-21 in cisplatin resistant ovarian cancer via JNK-1/c-Jun pathway - PubMed (original) (raw)
Upregulation of miR-21 in cisplatin resistant ovarian cancer via JNK-1/c-Jun pathway
Ileabett M Echevarría-Vargas et al. PLoS One. 2014.
Erratum in
- PLoS One. 2014;9(12):e116447
Abstract
Cisplatin has been the most accepted drug for the treatment of ovarian cancer for almost 40 years. Although the majority of patients with ovarian cancer respond to front-line platinum combination chemotherapy, many patients will develop cisplatin-resistance disease, which is extremely rapid and fatal. Although various mechanisms of cisplatin resistance have been postulated, the key molecules involved in such resistance have not been identified. MiRNAs are endogenously expressed small non-coding RNAs, which are evolutionarily conserved and function as post-transcriptional regulators of gene expression. Dysregulation of miRNAs have been associated with cancer initiation, progression and drug resistance. The oncogenic miRNA-21, one of the best-studied miRNAs, is upregulated in almost all human cancers. However, the regulation of miR-21 in cisplatin resistant ovarian cancer cells has not been assessed. In this study, we measured the miR-21 expression by real-time PCR and found upregulation of miR-21 in cisplatin resistant compared with cisplatin sensitive ovarian cancer cells. Chromatin immunoprecipitation studies demonstrated the association of the c-Jun transcription factor to the pri-mir-21 DNA promoter regions. Blocking the JNK-1, the major activator of c-Jun phosphorylation, reduced the expression of pre-mir-21 and increased the expression of its well-known target gene, PDCD4. Overexpression of miR-21 in cisplatin sensitive cells decreased PDCD4 levels and increased cell proliferation. Finally, targeting miR-21 reduced cell growth, proliferation and invasion of cisplatin resistant ovarian cancer cells. These results suggest that the JNK-1/c-Jun/miR-21 pathway contributes to the cisplatin resistance of ovarian cancer cells and demonstrated that miR-21 is a plausible target to overcome cisplatin resistance.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exist.
Figures
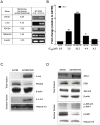
Figure 1. RT-PCR and western blot analysis of miRNA-21-related molecules.
(A) Validation of microarrays by RT-PCR. (B) MiR-21 levels in a panel of ovarian cancer cells. MiR-21 levels were expressed relative to the A2780 cells miR-21 levels. IC50s were calculated after 72-hr treatment of cells with different concentrations of cisplatin as described in the “Material and Methods” section. (C) Evaluation of c-Jun and p-c-Jun protein expression in A2780 and A2780CP20 cells. (D) Protein expression analysis of MAPKs in total and nuclear fractions of A2780 and A2780CP20 cells. Expression level in Figures A, C and D are without cisplatin treatment. *p<0.05, **p<0.01, ***p<0.001 compared to control. Columns represent the means of triplicates ± S.E.M.
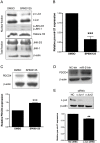
Figure 2. Effect of JNK-1 inhibition in miR-21 and PDCD4 expression.
A2780CP20 cells were treated with 10 µM SP600125. (A) Western blot shown the inhibition of p-c-Jun after treatment with SP600125 in A2780CP20 cells compared to control (DMSO). (B) SYBR-I-based real-time PCR was performed to calculate the relative pre-mir-21 expression in A2780CP20 cells after treatment with SP600125 inhibitor. (C) Western blot and densitometric analysis of PDCD4 protein expression levels after treatment of A2780CP20 cells with SP600125. (D) PDCD4 protein expression levels after transfection of A2780CP20 with miR-21 oligonucleotide inhibitor. (E) A2780 CP20 cells were transiently transfected with two c-Jun-targeted siRNAs as described in the “Materials and Methods” section. Western blot analysis shows that both c-Jun-siRNAs decreased the c-Jun levels. SYBR-I-based real-time PCR was performed (see “Materials and Methods” section) to calculate the relative pre-mir-21 expression levels in A2780CP20 cells after siRNA-mediated c-Jun silencing. *p<0.05, **p<0.01, ***p<0.001 compared to control. Columns represent the means of triplicates ± S.E.M.
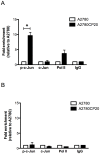
Figure 3. Chromatin immunoprecipitation assay (ChIP).
ChIP assay was performed as described in the “Materials and Methods” section. (A) SYBR-I-based real-time PCR amplification of the region containing the c-Jun recognition sequence in the pri-miR-21 DNA. The phospho-c-Jun levels bound to the pri-miR-21 promoter was higher in A2780CP20 cells compared with A2780 cells. (B) SYBR-I-based real-time PCR amplification of a DNA region far of the pre-mir-21 promoter was performed as a control. *p<0.05 compared to control. Columns represent the means of triplicates ± S.E.M.
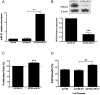
Figure 4. Effect of pre-mir-21 overexpression in A2780 cells.
(A) A2780 cells were stably transfected with pCMV-miR21 or empty pCMV-EV vectors. The miR-21 expression was quantified by qRT-PCR. (B) Western blot analysis shows a decreased expression of PDCD4 levels in A2780-miR-21 compared with A2780-EV cells. (C) Overexpression of miR21 increased cell proliferation (13%, ***p<0.001) in A2780-miR-21 compared with A2780-EV cells. (D) A2780-miR-21 overexpressed clones were more resistant to cisplatin-induced cell death compared with untransfected A2780 cells or with the A2780-EV cells. **p<0.01. Columns represent the means of triplicates ± S.E.M.
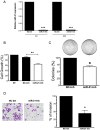
Figure 5. Effect of miR-21 inhibition in cell growth, proliferation and invasion.
(A) A2780CP20 cells were transiently transfected with a miR-21 antagomir or with a negative antagomir (-) as described in the “Materials and Methods” section. Eight and 24 hours after transfection cells were collected and RNA (including miRNAs) was isolated as described in the “Materials and Methods” section. (B) MiR-21 inhibition reduced cell growth compared to untreated cells (NT) or with the negative control inhibitor (NC-Inh). (C) A2780CP20 cells were transfected as in 5A. A thousand cells were seeded in Petri dishes. Ten days later, the colonies were stained and counted. Inhibition of miR-21 decreased the ability of cells to undergo unlimited division compared with the NC-Inh. (D) Cell invasion was carried out as described in the “Materials and Methods” section. The number of invaded cells was expressed in percentages, taken the NC-Inh as 100%. *p<0.05, **p<0.01 compared to NC-Inh. Columns represent the means of triplicates ± S.E.M.
Similar articles
- Recovery of miR-139-5p in Ovarian Cancer Reverses Cisplatin Resistance by Targeting C-Jun.
Jiang Y, Jiang J, Jia H, Qiao Z, Zhang J. Jiang Y, et al. Cell Physiol Biochem. 2018;51(1):129-141. doi: 10.1159/000495169. Epub 2018 Nov 15. Cell Physiol Biochem. 2018. PMID: 30439707 - Berberine sensitizes ovarian cancer cells to cisplatin through miR-21/PDCD4 axis.
Liu S, Fang Y, Shen H, Xu W, Li H. Liu S, et al. Acta Biochim Biophys Sin (Shanghai). 2013 Sep;45(9):756-62. doi: 10.1093/abbs/gmt075. Epub 2013 Jul 3. Acta Biochim Biophys Sin (Shanghai). 2013. PMID: 23824073 - Downregulation of miR-130a contributes to cisplatin resistance in ovarian cancer cells by targeting X-linked inhibitor of apoptosis (XIAP) directly.
Zhang X, Huang L, Zhao Y, Tan W. Zhang X, et al. Acta Biochim Biophys Sin (Shanghai). 2013 Dec;45(12):995-1001. doi: 10.1093/abbs/gmt113. Epub 2013 Oct 20. Acta Biochim Biophys Sin (Shanghai). 2013. PMID: 24145606 - miRNAs and ovarian cancer: a miRiad of mechanisms to induce cisplatin drug resistance.
Samuel P, Pink RC, Brooks SA, Carter DR. Samuel P, et al. Expert Rev Anticancer Ther. 2016;16(1):57-70. doi: 10.1586/14737140.2016.1121107. Epub 2015 Dec 5. Expert Rev Anticancer Ther. 2016. PMID: 26567444 Review. - Targeting ferroptosis in ovarian cancer: Novel strategies to overcome chemotherapy resistance.
Kapper C, Oppelt P, Arbeithuber B, Gyunesh AA, Vilusic I, Stelzl P, Rezk-Füreder M. Kapper C, et al. Life Sci. 2024 Jul 15;349:122720. doi: 10.1016/j.lfs.2024.122720. Epub 2024 May 16. Life Sci. 2024. PMID: 38762066 Review.
Cited by
- CEA, CA 15-3, and miRNA expression as potential biomarkers in canine mammary tumors.
Jain M, Ingole SD, Deshmukh RS, Bharucha SV, Nagvekar AS, Gaikwad RV, Kharde SD. Jain M, et al. Chromosome Res. 2021 Jun;29(2):175-188. doi: 10.1007/s10577-021-09652-7. Epub 2021 Feb 27. Chromosome Res. 2021. PMID: 33638118 - Metabolic Remodeling as a Way of Adapting to Tumor Microenvironment (TME), a Job of Several Holders.
Serpa J. Serpa J. Adv Exp Med Biol. 2020;1219:1-34. doi: 10.1007/978-3-030-34025-4_1. Adv Exp Med Biol. 2020. PMID: 32130691 Review. - Effect of Cyclodextrins Formulated in Liposomes and Gold and Selenium Nanoparticles on siRNA Stability in Cell Culture Medium.
Castillo Cruz B, Chinapen Barletta S, Ortiz Muñoz BG, Benitez-Reyes AS, Amalbert Perez OA, Cardona Amador AC, Vivas-Mejia PE, Barletta GL. Castillo Cruz B, et al. Pharmaceuticals (Basel). 2024 Oct 8;17(10):1344. doi: 10.3390/ph17101344. Pharmaceuticals (Basel). 2024. PMID: 39458985 Free PMC article. - Small Non-Coding-RNA in Gynecological Malignancies.
Dwivedi SKD, Rao G, Dey A, Mukherjee P, Wren JD, Bhattacharya R. Dwivedi SKD, et al. Cancers (Basel). 2021 Mar 3;13(5):1085. doi: 10.3390/cancers13051085. Cancers (Basel). 2021. PMID: 33802524 Free PMC article. Review. - Interactions between anticancer active platinum complexes and non-coding RNAs/microRNAs.
Biersack B. Biersack B. Noncoding RNA Res. 2016 Oct 13;2(1):1-17. doi: 10.1016/j.ncrna.2016.10.001. eCollection 2017 Mar. Noncoding RNA Res. 2016. PMID: 30159416 Free PMC article. Review.
References
- Siegel R, Naishadham D, Jemal A (2013) Cancer Statistics, 2013. 63: 11–30. - PubMed
- Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, et al. (2011) Molecular mechanisms of cisplatin resistance. Oncogene 1869–1883. - PubMed
- Kikuchi Y (2001) [The mechanism of cisplatin-resistance in ovarian cancer]. Human cell 14: 115–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1K22CA166226-01A1/CA/NCI NIH HHS/United States
- R25 GM061838/GM/NIGMS NIH HHS/United States
- K22 CA166226/CA/NCI NIH HHS/United States
- R25-GM061838/GM/NIGMS NIH HHS/United States
- U54 MD008149/MD/NIMHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous