Pretreatment blood-brain barrier damage and post-treatment intracranial hemorrhage in patients receiving intravenous tissue-type plasminogen activator - PubMed (original) (raw)
Comparative Study
Pretreatment blood-brain barrier damage and post-treatment intracranial hemorrhage in patients receiving intravenous tissue-type plasminogen activator
Richard Leigh et al. Stroke. 2014 Jul.
Abstract
Background and purpose: Early blood-brain barrier damage after acute ischemic stroke has previously been qualitatively linked to subsequent intracranial hemorrhage (ICH). In this quantitative study, it was investigated whether the amount of blood-brain barrier damage evident on pre-tissue-type plasminogen activator MRI scans was related to the degree of post-tissue-type plasminogen activator ICH in patients with acute ischemic stroke.
Methods: Analysis was performed on a database of patients with acute ischemic stroke provided by the Stroke Imaging Repository (STIR) and Virtual International Stroke Trials Archive (VISTA) Imaging Investigators. Patients with perfusion-weighted imaging lesions>10 mL and negative gradient-recalled echo imaging before intravenous tissue-type plasminogen activator were included. Postprocessing of the perfusion-weighted imaging source images was performed to estimate changes in blood-brain barrier permeability within the perfusion deficit relative to the unaffected hemisphere. Follow-up gradient-recalled echo images were reviewed for evidence of ICH and divided into 3 groups according to European Cooperative Acute Stroke Study (ECASS) criteria: no hemorrhage, hemorrhagic infarction, and parenchymal hematoma.
Results: Seventy-five patients from the database met the inclusion criteria, 28 of whom experienced ICH, of which 19 were classified as hemorrhagic infarction and 9 were classified as parenchymal hematoma. The mean permeability (±SDs), expressed as an index of contrast leakage, was 17.0±8.8% in the no hemorrhage group, 19.4±4.0% in the hemorrhagic infarction group, and 24.6±4.5% in the parenchymal hematoma group. Permeability was significantly correlated with ICH grade in univariate (P=0.007) and multivariate (P=0.008) linear regression modeling.
Conclusions: A perfusion-weighted imaging-derived index of blood-brain barrier damage measured before intravenous tissue-type plasminogen activator is given is associated with the severity of ICH after treatment in patients with acute ischemic stroke.
Keywords: blood–brain barrier; magnetic resonance imaging; stroke.
© 2014 American Heart Association, Inc.
Conflict of interest statement
Conflicts-of-interest/Disclosures:
Peter B. Barker has served as a consultant to Olea Medical.
Figures
Figure 1
Example of mean permeability ROI: The TTP map (A) is thresholded (B) and overlain on the permeability map (C).
Figure 2
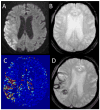
Example images for a patient who suffered parenchymal hematoma after tPA are shown. Panel A: pre-treatment DWI. Panel B: pre-treatment GRE. Panel C: pre-treatment permeability image. Panel D: post-treatment GRE demonstrating ICH.
Figure 3
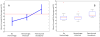
Panel A shows the mean permeability derangement for each group with 95% confidence intervals. The red line demonstrates a threshold that separates parenchymal hematoma from all other patients with 95% accuracy. Panel B shows box plots for the mean permeability derangement of each group; the central mark is the median, the edges of the box are the 25th and 75th percentiles, the bars extend to the most extreme data points not considered outliers, and outliers are plotted individually.
Similar articles
- Computed Tomography Perfusion Derived Blood-Brain Barrier Permeability Does Not Yet Improve Prediction of Hemorrhagic Transformation.
Horsch AD, Bennink E, van Seeters T, Kappelle LJ, van der Graaf Y, Mali WPTM, de Jong HWAM, Velthuis BK, Dankbaar JW; DUST Investigators. Horsch AD, et al. Cerebrovasc Dis. 2018;45(1-2):26-32. doi: 10.1159/000485043. Epub 2018 Jan 8. Cerebrovasc Dis. 2018. PMID: 29402765 Free PMC article. - Two tales: hemorrhagic transformation but not parenchymal hemorrhage after thrombolysis is related to severity and duration of ischemia: MRI study of acute stroke patients treated with intravenous tissue plasminogen activator within 6 hours.
Thomalla G, Sobesky J, Köhrmann M, Fiebach JB, Fiehler J, Zaro Weber O, Kruetzelmann A, Kucinski T, Rosenkranz M, Röther J, Schellinger PD. Thomalla G, et al. Stroke. 2007 Feb;38(2):313-8. doi: 10.1161/01.STR.0000254565.51807.22. Epub 2007 Jan 4. Stroke. 2007. PMID: 17204683 - Symptomatic intracranial hemorrhage following intravenous thrombolysis for acute ischemic stroke: a critical review of case definitions.
Seet RC, Rabinstein AA. Seet RC, et al. Cerebrovasc Dis. 2012;34(2):106-14. doi: 10.1159/000339675. Epub 2012 Aug 1. Cerebrovasc Dis. 2012. PMID: 22868870 Review. - Risk factors for intracranial hemorrhage in acute ischemic stroke patients treated with recombinant tissue plasminogen activator: a systematic review and meta-analysis of 55 studies.
Whiteley WN, Slot KB, Fernandes P, Sandercock P, Wardlaw J. Whiteley WN, et al. Stroke. 2012 Nov;43(11):2904-9. doi: 10.1161/STROKEAHA.112.665331. Epub 2012 Sep 20. Stroke. 2012. PMID: 22996959 Review.
Cited by
- D1 receptor-mediated endogenous tPA upregulation contributes to blood-brain barrier injury after acute ischaemic stroke.
Wang Y, Wang X, Zhang X, Chen S, Sun Y, Liu W, Jin X, Zheng G. Wang Y, et al. J Cell Mol Med. 2020 Aug;24(16):9255-9266. doi: 10.1111/jcmm.15570. Epub 2020 Jul 6. J Cell Mol Med. 2020. PMID: 32627929 Free PMC article. - β2-Adrenergic Receptor-Mediated HIF-1α Upregulation Mediates Blood Brain Barrier Damage in Acute Cerebral Ischemia.
Sun Y, Chen X, Zhang X, Shen X, Wang M, Wang X, Liu WC, Liu CF, Liu J, Liu W, Jin X. Sun Y, et al. Front Mol Neurosci. 2017 Aug 14;10:257. doi: 10.3389/fnmol.2017.00257. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28855859 Free PMC article. - The Relationship Between Penumbral Tissue and Blood-Brain Barrier Disruption in Acute Stroke Patients Presenting in an Extended Time Window.
Heidari P, Blayney S, Butler J, Hitomi E, Luby M, Leigh R. Heidari P, et al. Front Neurol. 2020 Dec 8;11:582994. doi: 10.3389/fneur.2020.582994. eCollection 2020. Front Neurol. 2020. PMID: 33363505 Free PMC article. - Fibrinogen Concentrate for the Treatment of Thrombolysis-Associated Hemorrhage in Adult Ischemic Stroke Patients.
Barra ME, Feske SK, Sylvester KW, Ong C, Culbreth SE, Krause P, Henderson GV, Rybak E. Barra ME, et al. Clin Appl Thromb Hemost. 2020 Jan-Dec;26:1076029620951867. doi: 10.1177/1076029620951867. Clin Appl Thromb Hemost. 2020. PMID: 32946279 Free PMC article. - A Review of Mathematics Determining Solute Uptake at the Blood-Brain Barrier in Normal and Pathological Conditions.
Sprowls SA, Saralkar P, Arsiwala T, Adkins CE, Blethen KE, Pizzuti VJ, Shah N, Fladeland R, Lockman PR. Sprowls SA, et al. Pharmaceutics. 2021 May 19;13(5):756. doi: 10.3390/pharmaceutics13050756. Pharmaceutics. 2021. PMID: 34069733 Free PMC article. Review.
References
- The national institute of neurological disorders and stroke rt-pa stroke study group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. - PubMed
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. - PubMed
- Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von KR, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The european cooperative acute stroke study (ecass) JAMA. 1995;274:1017–1025. - PubMed
- The ninds t-pa stroke study group. Intracerebral hemorrhage after intravenous t-pa therapy for ischemic stroke. Stroke. 1997;28:2109–2118. - PubMed
- Hjort N, Wu O, Ashkanian M, Solling C, Mouridsen K, Christensen S, et al. Mri detection of early blood-brain barrier disruption: Parenchymal enhancement predicts focal hemorrhagic transformation after thrombolysis. Stroke. 2008;39:1025–1028. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01NS47691/NS/NINDS NIH HHS/United States
- R01 NS047691/NS/NINDS NIH HHS/United States
- P41 RR015241/RR/NCRR NIH HHS/United States
- K24 NS072272/NS/NINDS NIH HHS/United States
- R01DC05375/DC/NIDCD NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- R01 DC005375/DC/NIDCD NIH HHS/United States
- P41 EB015909/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous