Sulforaphane reduces vascular inflammation in mice and prevents TNF-α-induced monocyte adhesion to primary endothelial cells through interfering with the NF-κB pathway - PubMed (original) (raw)
doi: 10.1016/j.jnutbio.2014.03.011. Epub 2014 Apr 4.
Hongwei Si 2, Pon Velayutham Anandh Babu 3, Dengke Pan 4, Yu Fu 5, Elizabeth A S Brooke 1, Halley Shah 1, Wei Zhen 5, Hong Zhu 6, Dongmin Liu 7, Yunbo Li 8, Zhenquan Jia 9
Affiliations
- PMID: 24880493
- PMCID: PMC4087147
- DOI: 10.1016/j.jnutbio.2014.03.011
Sulforaphane reduces vascular inflammation in mice and prevents TNF-α-induced monocyte adhesion to primary endothelial cells through interfering with the NF-κB pathway
Palanisamy Nallasamy et al. J Nutr Biochem. 2014 Aug.
Abstract
Sulforaphane, a naturally occurring isothiocyanate present in cruciferous vegetables, has received wide attention for its potential to improve vascular function in vitro. However, its effect in vivo and the molecular mechanism of sulforaphane at physiological concentrations remain unclear. Here, we report that a sulforaphane concentration as low as 0.5 μM significantly inhibited tumor necrosis factor-α (TNF-α)-induced adhesion of monocytes to human umbilical vein endothelial cells, a key event in the pathogenesis of atherosclerosis both in static and under flow conditions. Such physiological concentrations of sulforaphane also significantly suppressed TNF-α-induced production of monocyte chemotactic protein-1 and adhesion molecules including soluble vascular adhesion molecule-1 and soluble E-selectin, key mediators in the regulation of enhanced endothelial cell-monocyte interaction. Furthermore, sulforaphane inhibited TNF-α-induced nuclear factor (NF)-κB transcriptional activity, Inhibitor of NF-κB alpha (IκBα) degradation and subsequent NF-κB p65 nuclear translocation in endothelial cells, suggesting that sulforaphane can inhibit inflammation by suppressing NF-κB signaling. In an animal study, sulforaphane (300 ppm) in a mouse diet significantly abolished TNF-α-increased ex vivo monocyte adhesion and circulating adhesion molecules and chemokines in C57BL/6 mice. Histology showed that sulforaphane treatment significantly prevented the eruption of endothelial lining in the intima layer of the aorta and preserved elastin fibers' delicate organization, as shown by Verhoeff-van Gieson staining. Immunohistochemistry studies showed that sulforaphane treatment also reduced vascular adhesion molecule-1 and monocyte-derived F4/80-positive macrophages in the aorta of TNF-α-treated mice. In conclusion, sulforaphane at physiological concentrations protects against TNF-α-induced vascular endothelial inflammation, in both in vitro and in vivo models. This anti-inflammatory effect of sulforaphane may be, at least in part, associated with interfering with the NF-κB pathway.
Keywords: In vivo; Physiological concentrations; Sulforaphane; TNF-α; Vascular inflammation.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest: None declared.
Figures
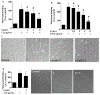
Fig. 1. Sulforaphane inhibited TNF-α-stimulated monocyte adhesion to HUVECs in static and shear flow conditions
(A–C) Sulforaphane inhibited TNF-α-induced monocyte adhesion to ECs in static conditions. HUVECs were pre-treated with various concentrations of sulforaphane (S) for 1 h before addition of TNF-α (T 2 ng/mL) in the presence or absence of sulforaphane for 6 h. THP-1 cells were labeled with a fluorescence probe and the adhesion was determined using a Microplate Reader at excitation and emission wavelengths of 496 nm and 520 nm (A). Images were captured using a florescence microscope (B) or a Nikon Phase contrast microscope (D). Values are mean ± SEM, n=4. (D) TNF-α-induced monocyte adhesion to ECs under shear flow control. Values are mean ± SEM, n=3. *, p<0.05 vs. control; #, p<0.05 vs. TNF-α alone-treated cells.
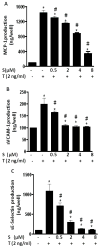
Fig. 2. Sulforaphane suppressed the production of MCP-1 (A), sVCAM-1 (B) and sE-Selectin (C) in HUVECs
HUVECs were pre-treated with various concentrations of sulforaphane (S) for 1 h before addition of TNF-α (T 2 ng/mL) in the presence or absence of sulforaphane for 6 h. MCP-1, sVCAM-1 and sE-Selectin were measured by ELISA. Data are expressed as mean ± SEM from three experiments. *, p<0.05 vs. control; #, p<0.05 vs. TNF-α alone-treated cells. MCP-1, monocyte chemoattractant protein -1; sVCAM-1, soluble vascular adhesion molecule-1; sE-Selectin, soluble E-Selectin.
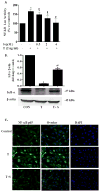
Fig. 3. Sulforaphane inhibited TNF-α-induced NF-κB signaling in ECs
(A) The effect of sulforaphane on TNF-α-induced NF-κB transcriptional activity. ECs were co-transfected with NF-κB promoter-luciferase vector and pRL reporter control plasmid. 24 h after transfection, ECs were treated with sulforaphane for 1 h before addition of TNF-α (2 ng/mL) for 6 h. Luciferase activity, normalized to pRL activity in the cell extracts, was determined. Values are mean ± SEM, n=3. (B) IκBα protein level was determined by Western blot analysis. β-actin was used as a loading control. Values are mean ± SE, n=3. (c) NF-κB p65 nuclear translocation was visualized using immunofluorescence staining. Representative sections are shown NF-κB p65, overlay and DAPI. *, p<0.05 vs. control; #, p<0.05 vs. TNF-α alone-treated cells.
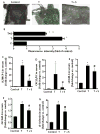
Fig. 4. Dietary sulforaphane reduced the monocyte binding to aortic endothelium (A–B), the secretion of serum chemokines (C–D) and adhesion molecules (E–G) in TNF-α treated mice
Values are mean ± SEM, n=8–10. *, p<0.05 vs. control; #, p<0.05 vs. TNF-α alone-treated mice. sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular adhesion molecule-1; sE-Selectin, soluble E-Selectin; MCP-1/JE, mouse monocyte chemotactic protein 1/JE; TNF-α, Tumor necrosis factor-α; CXCL1/KC, Chemokine (C-X-C motif) ligand 1.
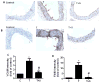
Fig 5. Immunohistochemical staining for adhesion molecule VCAM-1 and F4/80-positive monocytes-derived macrophages in aortic cross-sections
Representative photomicrographs of immunohistochemical staining for F4/80-positive monocytes-derived macrophages (A) and VCAM-1 (B). Quantitative analysis of VCAM-1 (C) and F4/80 (D). Arrows indicate typical positive-stained regions and original magnification is 40X. T, TNF-α; T +S, TNF-α + sulforaphane. Data are expressed as mean ± SEM, n=5, *, p<0.05 vs. control; #, p<0.05 vs. TNF-α alone-treated mice.
Fig. 6. Dietary sulforaphane prevented TNF-α-induced aortic endothelial injury (A) and disruption of aortic elastin fiber (B) in aortic cross sections of TNF-α treated mice
Representative histological sections of aorta for hematoxylin and eosin staining (A) and Verhoeff-Van Gieson staining (B). Aortic sections were stained with hematoxylin and eosin and Verhoeff-Van Gieson staining as described in the Materials and Methods section.
Similar articles
- Luteolin protects against vascular inflammation in mice and TNF-alpha-induced monocyte adhesion to endothelial cells via suppressing IΚBα/NF-κB signaling pathway.
Jia Z, Nallasamy P, Liu D, Shah H, Li JZ, Chitrakar R, Si H, McCormick J, Zhu H, Zhen W, Li Y. Jia Z, et al. J Nutr Biochem. 2015 Mar;26(3):293-302. doi: 10.1016/j.jnutbio.2014.11.008. Epub 2014 Dec 15. J Nutr Biochem. 2015. PMID: 25577468 Free PMC article. - Natural Compound Resveratrol Attenuates TNF-Alpha-Induced Vascular Dysfunction in Mice and Human Endothelial Cells: The Involvement of the NF-κB Signaling Pathway.
Nallasamy P, Kang ZY, Sun X, Anandh Babu PV, Liu D, Jia Z. Nallasamy P, et al. Int J Mol Sci. 2021 Nov 19;22(22):12486. doi: 10.3390/ijms222212486. Int J Mol Sci. 2021. PMID: 34830366 Free PMC article. - ZLJ-6, a novel COX/5-LOX inhibitor, attenuates TNF-α-induced endothelial E-selectin, ICAM-1 and VCAM-1 expression and monocyte-endothelial interactions via a COX/5-LOX-independent mechanism.
Chen L, Zhao Q, Wang XL, You R, Zhang YH, Ji H, Lai YS. Chen L, et al. Vascul Pharmacol. 2011 Nov-Dec;55(5-6):135-42. doi: 10.1016/j.vph.2011.07.003. Epub 2011 Jul 12. Vascul Pharmacol. 2011. PMID: 21777697 - Monocyte-endothelial cell interactions in the development of atherosclerosis.
Mestas J, Ley K. Mestas J, et al. Trends Cardiovasc Med. 2008 Aug;18(6):228-32. doi: 10.1016/j.tcm.2008.11.004. Trends Cardiovasc Med. 2008. PMID: 19185814 Free PMC article. Review. - Activated monocytes as a therapeutic target to attenuate vascular inflammation and lower cardiovascular disease-risk in patients with type 2 diabetes: A systematic review of preclinical and clinical studies.
Ngcobo SR, Nkambule BB, Nyambuya TM, Mokgalaboni K, Ntsethe A, Mxinwa V, Ziqubu K, Ntamo Y, Nyawo TA, Dludla PV. Ngcobo SR, et al. Biomed Pharmacother. 2022 Feb;146:112579. doi: 10.1016/j.biopha.2021.112579. Epub 2021 Dec 30. Biomed Pharmacother. 2022. PMID: 35062054
Cited by
- The Immunomodulatory Effects of Sulforaphane in Exercise-Induced Inflammation and Oxidative Stress: A Prospective Nutraceutical.
Ruhee RT, Suzuki K. Ruhee RT, et al. Int J Mol Sci. 2024 Feb 1;25(3):1790. doi: 10.3390/ijms25031790. Int J Mol Sci. 2024. PMID: 38339067 Free PMC article. Review. - Sulforaphane Attenuates Muscle Inflammation in Dystrophin-deficient mdx Mice via NF-E2-related Factor 2 (Nrf2)-mediated Inhibition of NF-κB Signaling Pathway.
Sun CC, Li SJ, Yang CL, Xue RL, Xi YY, Wang L, Zhao QL, Li DJ. Sun CC, et al. J Biol Chem. 2015 Jul 17;290(29):17784-17795. doi: 10.1074/jbc.M115.655019. Epub 2015 May 26. J Biol Chem. 2015. PMID: 26013831 Free PMC article. Retracted. - Suberanilohydroxamic Acid as a Pharmacological Kruppel-Like Factor 2 Activator That Represses Vascular Inflammation and Atherosclerosis.
Xu Y, Xu S, Liu P, Koroleva M, Zhang S, Si S, Jin ZG. Xu Y, et al. J Am Heart Assoc. 2017 Nov 30;6(12):e007134. doi: 10.1161/JAHA.117.007134. J Am Heart Assoc. 2017. PMID: 29191808 Free PMC article. - The saponin DT-13 attenuates tumor necrosis factor-α-induced vascular inflammation associated with Src/NF-кB/MAPK pathway modulation.
Zhang Y, Sun M, Han Y, Zhai K, Tang Y, Qin X, Cao Z, Yu B, Kou J. Zhang Y, et al. Int J Biol Sci. 2015 Jun 11;11(8):970-81. doi: 10.7150/ijbs.11635. eCollection 2015. Int J Biol Sci. 2015. PMID: 26157351 Free PMC article. - TNF-α is a Novel Biomarker for Predicting Plaque Rupture in Patients with ST-Segment Elevation Myocardial Infarction.
Luo X, Zhao C, Wang S, Jia H, Yu B. Luo X, et al. J Inflamm Res. 2022 Mar 14;15:1889-1898. doi: 10.2147/JIR.S352509. eCollection 2022. J Inflamm Res. 2022. PMID: 35313673 Free PMC article.
References
- Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annual review of pathology. 2006;1:297–329. - PubMed
- Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clinical chemistry. 2008;54:24–38. - PubMed
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. - PubMed
- Mayer K, Merfels M, Muhly-Reinholz M, Gokorsch S, Rosseau S, Lohmeyer J, et al. Omega- 3 fatty acids suppress monocyte adhesion to human endothelial cells: role of endothelial PAF generation. Am J Physiol Heart Circ Physiol. 2002;283:H811–8. - PubMed
- Chen JW, Chen YH, Lin FY, Chen YL, Lin SJ. Ginkgo biloba extract inhibits tumor necrosis factor-alpha-induced reactive oxygen species generation, transcription factor activation, and cell adhesion molecule expression in human aortic endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:1559–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AT007077/AT/NCCIH NIH HHS/United States
- R15 AT005372/AT/NCCIH NIH HHS/United States
- 1R01AT007077-01/AT/NCCIH NIH HHS/United States
- 1R15AT005372/AT/NCCIH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials