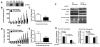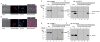Effects of tumor-suppressor lysyl oxidase propeptide on prostate cancer xenograft growth and its direct interactions with DNA repair pathways - PubMed (original) (raw)
Effects of tumor-suppressor lysyl oxidase propeptide on prostate cancer xenograft growth and its direct interactions with DNA repair pathways
M V Bais et al. Oncogene. 2015.
Abstract
Lysyl oxidase (LOX) is a multifunctional protein required for normal collagen and elastin biosynthesis and maturation. In addition, LOX has complex roles in cancer in which the lysyl oxidase propeptide (LOX-PP) domain of secreted pro-LOX has tumor-suppressor activity, while the active enzyme promotes metastasis. In prostate cancer cell lines, recombinant LOX-PP (rLOX-PP) inhibits the growth of PC3 cells in vitro by mechanisms that were not characterized, while in DU145 cells rLOX-PP targeted fibroblast growth factor signaling. Because rLOX-PP can enhance effects of a genotoxic chemotherapeutic on breast cancer cell apoptosis, we reasoned that rLOX-PP could target DNA repair pathways typically elevated in cancer. Here we demonstrate for the first time that rLOX-PP inhibits prostate xenograft growth in vivo and that activating phosphorylations of the key DNA repair molecules ataxia-telangiectasia mutated (ATM) and checkpoint kinase 2 (CHK2) are inhibited by rLOX-PP expression in vivo. In addition, in vitro studies showed that rLOX-PP inhibits radiation-induced activating phosphorylations of ATM and CHK2 and that exogenously added rLOX-PP protein can localize to the nucleus in both DU145 and PC3 cells. rLOX-PP pull-down studies resulted in detection of a protein complex with the nuclear DNA repair regulator MRE11 in both cell lines, and rLOX-PP localized to radiation-induced nuclear DNA repair foci. Finally, rLOX-PP was shown to sensitize both DU145 and PC3 cells to radiation-induced cell death determined in colony-formation assays. These data provide evidence that rLOX-PP has a nuclear mechanism of action in which it directly interacts with DNA repair proteins to sensitize prostate cancer cells to the effects of ionizing radiation.
Conflict of interest statement
All authors declare that they have no conflicts of interest regarding the contents of this manuscript.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Figures
Figure 1. Ectopic overexpression of LOX-PP inhibits DU145 and PC3 xenografts growth in mice and inhibits ATM and CHK2 phosphorylation
(A) Ectopic overexpression of rLOX-PP in PC3 and DU145 cells by lentiviral transduction as described in Materials and Methods, and Western blot analysis of cell culture supernatants to evaluate LOX-PP expression. Lanes rL1 and rL5 are 1 and 5 ng of rLOX-PP protein as a Western blot control; lane DE, conditioned medium sample from DU145 cells transduced with CMV-Empty; lane DL, DU145 conditioned medium sample from cells transduced with rLOX-PP lentivirus; lane PE, conditioned medium sample from PC3 cells transduced with Empty lentivirus particles; lane PL, conditioned medium sample from PC3 cells transduced with rLOX-PP lentivirus; (B) subcutaneous murine xenografts of PC3 cells infected with Empty or rLOX-PP expressing virus as generated above. The tumor size was monitored by caliper measurements (n=5); * p<0.05 analysis by two way ANOVA; (C) tumor weight at sacrifice on day 70. (D) Subcutaneous murine xenografts of DU145 cells infected with Empty or rLOX-PP expressing lentivirus particles (n=5); * p<0.05, ** p<0.01, *** p<0.001; analysis by two way ANOVA; (E) tumor weight at sacrifice on day 52; (F) Western blot analysis of tumor extracts for ATM and CHK2 phosphorylation, total ATM and CHK2 and β-actin. Equal amounts of protein from each tumor xenograft (n=4) were pooled together and 40 μg samples were loaded to evaluate the expression of phospho-ATM/CHK2, total ATM/CHK2 and β-actin. These pooled samples were run 3 times, quantified and plotted. (G) Quantification and normalization of phospho-ATM and CHK2 levels from tumors derived from Western blots. Data are the averages of means of three independent experiments +/− SEM; n = 3, * p<0.05, ** p<0.01, Student’s t-test.
Figure 2. LOX-PP inhibits IR induced DNA damage repair response by inhibiting ATM phosphorylation
PC3 and DU145 cells transduced with Empty and rLOX-PP lentivirus particles were plated in 6-well plates and subjected to IR (5 Gy). Equal amounts of protein extracts from each cell sample (n=3) were pooled together and 40 μg samples were evaluated for the expression of phospho-ATM, total ATM and β-actin by Western blotting. The experiments were repeated with 3 different preparations of cells. (A) Representative Western blots for phospho-ATM, total ATM and β-actin as a loading control from PC3-Empty and PC3-LOX-PP cells subjected to IR (5 Gy) (B) quantification of relative protein expression in experimental groups quantified by densitometry analysis; (C) representative Western blots for phospho-ATM, total ATM and β-actin as a loading control from DU145-Empty and DU145-LOX-PP cells subjected to IR (5 Gy); (D) the quantification of relative protein expression quantified by densitometry analysis; The experiments were repeated 3 times. Data are the averages of means of three independent experiments +/− SEM (n=3; * P<0.05, **p<0.01, *** P<0.001; student’s t-test).
Figure 3. LOX-PP inhibits DNA damage induced CHK2 phosphorylation and DNA fragmentation detected by agarose gel electrophoresis
PC3 and DU145 cells were transduced with Empty- and rLOX-PP-expressing lentivirus particles and were cultured in 6-well plates and subjected to IR (5 Gy). Equal amounts of cell layer protein extracts from each cell extract (n=3) were pooled together and 40 μg aliquots of protein were evaluated for the expression of phospho-CHK2, total CHK2 and β-actin. The experiments were performed 3 times with different preparations of cells. (A) Representative Western blots for phospho-CHK2, total CHK2 and β-actin as a loading control from the PC3-Empty and PC3-LOX-PP cells subjected to IR (5 Gy); (B) the quantification of relative protein expression by densitometry analysis; (C) Western blot for phospho-CHK2, total CHK2 and β-actin as a loading control from the DU145-Empty and DU145-LOX-PP cells subjected to IR (5 Gy); (D) the quantification of relative protein expression by densitometry analysis. Experiments were performed 3 times. Data are the averages of means of three independent experiments +/− SEM (n=3; *, P<0.05; student’s t-test). (E) PC3 and DU145 cells were transduced with Empty and rLOX-PP-expressing lentivirus particles, were cultured in 6-well plates and subjected to IR (5 Gy). Cells were harvested after 24 hrs for DNA isolation and subjected to 2% agarose gel electrophoresis in the presence of ethidum bromide and then visualized under UV light and photographed; (M) molecular weight marker.
Figure 4. LOX-PP inhibition of IR induced DNA damage repair response is independent of its effect on Ras signaling pathways
PC3 and DU145 cells transduced with Empty and rLOX-PP-expressing lentivirus particles were plated in 6-well plates and subjected to IR (5 Gy). Equal amounts of protein extracts from each independent culture (n=3) were pooled and 40 μg samples were subjected to SDS PAGE and Western blotting to evaluate the expression of phospho-Akt, phospho ERK1/2, total AKT, total AKT and β-actin. The experiments were performed with 3 different preparations of cells. (A) Representative Western blots for phospho-AKT, total AKT and β-actin as a loading control and phospho ERK1/2, total ERK and β-actin from PC3-Empty and PC3-LOX-PP cells subjected to IR (5 Gy) (B) quantification of relative protein expression by densitometry analysis; (C) representative Western blots for phospho-AKT, total AKT and β-actin as a loading control and phospho ERK1/2, total ERK and β-actin from the PC3-Empty and PC3-LOX-PP cells from the DU145-Empty and DU145-LOX-PP cells subjected to IR (5 Gy) (D) the quantification of relative protein expression by densitometry analysis. Experiments were repeated 3 times. Data are the averages of means of three independent experiments +/− SEM (n=3).
Figure 5. rLOX-PP nuclear localization in prostate cancer cell line PC3 (A) and DU145 (B), and LOX-PP interaction with MRE11 protein complexes PC3 cells (C) and DU145 cells (D)
For uptake studies, 30,000 PC3 and DU145 cells in F12K or DMEM media were cultured in chamber slides in standard media and were then replenished with serum-free F12K or DMEM media (0.1% BSA, 1 % penicillin-streptomycin) for 24 hrs. rLOX-PP labeled with ATTO565 (4 μg/ml rLOX-PP-ATTO565) was added as indicated and incubated for 36 hrs, and subjected to confocal microscopy by using Zeiss 710 dual scanner confocal microscope in a live cell imaging chamber at 37°C with 5% CO2. (A and B) Confocal microscopy revealed apparent nuclear and cytoplasmic association of rLOX-PP in PC3 and DU145 cells, respectively. In each panel the images shown from left to right are phase contrast, rLOX-PP-ATTO565, Hoechst staining to label nuclei, and corresponding merged images showing in addition the 0.2 micrometer plane (Z-stack) derived from the indicated cross-section in the cell nucleus. For LOX-PP interaction studies (Panels C – F), PC3 (C and D) and DU145 (E and F) empty vector and rLOX-PP expressing PC3 and DU145 cells were cultured, extracted into non-denaturing cell lysis RIPA buffer. Cells were washed extensively with PBS, lysed and extracted with 500 μl non-denaturing cell lysis buffer (Profound c-Myc Tag IP/Co-IP kit; Thermo Scientific). Agarose beads with covalently bound non-immune IgG or anti-Myc-tag IgG antibody was added to samples and incubated overnight at 4° C to pull down rLOX-PP and any bound proteins. Resins were transferred to columns, washed, and then subjected to an acid elution protocol according to the manufacturer’s instructions. (C) Western blot analysis of 5% input samples from PC3-Empty and from PC3-LOX-PP cells in lanes 1 and 2 demonstrates the presence of MRE11. Eluted samples from non-immune IgG agarose in lanes 3 and 4 and from anti-Myc-tag IgG in lanes 5 and 6 identify MRE11 only from cells expressing rLOX-PP and only in the anti-Myc tag IgG agarose pull down, as expected; (D) detection of immunoprecipitated rLOX-PP after stripping and re-probing with anti-LOX-PP antibody showing endogenous 50 kDa pro-lysyl oxidase in lanes 7 and 8, and rLOX-PP in lanes 8 and 12 only. (E) Western blot analysis of 5% input samples from DU145-Empty and from DU145-LOX-PP cells in lanes 13 and 14 demonstrates the presence of MRE11. Eluted samples from non-immune IgG agarose in lanes 15 and 16 and from anti-Myc-tag IgG in lanes 17 and 18 identify MRE11 only from cells expressing rLOX-PP and only in the anti-Myc tag IgG agarose pull down, as expected; (F) detection of immunoprecipitated rLOX-PP after stripping and re-probing with anti-LOX-PP antibody identified rLOX-PP in lane 24 only, as expected. The experiments were repeated 3 times each from protein extracts from 3 different batches of cells with the same outcomes.
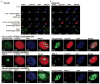
Figure 6. LOX-PP co-localizes with DNA repair foci in irradiated PC3 and DU145 cells
PC3 (A) or DU145 (B) cells were respectively cultured in chamber slides for 18 hrs in standard media with 10% serum, treated with labeled rLOX-PP (rLOX-PP-ATTO565) for 36 hrs for direct detection. Cells were subjected to 5 Gy IR. After 1 hour, irradiated and non-irradiated control cells were fixed, stained and subjected to confocal microscopy for rLOX-PP-ATTO565, phosphorylated-H2AX and MRE11 (Materials and Methods). Row A1, PC3 cells not treated with IR; row A2, PC3 cells no radiation control probed with anti-phosphorylated-H2AX; row A3, PC3 cells treated with IR in the presence of rLOX-PP-ATTO565, and probed with isotype non-immune antibody (negative control for anti-H2AX antibody), row A4, PC3 cells treated with IR in the absence of rLOX-PP-ATTO565, and probed with anti-phosphorylated-H2AX antibody; row A5, PC3 cells treated with IR and rLOX-PP-ATTO565, and probed with anti-phosphorylated-H2AX- and visualized for presence of rLOX-PP-ATTO565; row A6, PC3 cells treated with IR and rLOX-PP, and probed with MRE 11- and visualized for presence of rLOX-PP-ATTO565, and row A7, PC3 cells treated with IR and rLOX-PP-ATTO565, and probed with anti-phosphorylated-H2AX and MRE 11 antibody. Row B1; DU145 cells not treated with IR; row B2, DU145 cells not treated with IR but probed with anti-phosphorylated-H2AX antibody to detect endogenous expression of phosphorylated-H2AX independent of radiation treatment; row B3, DU145 cells treated with IR in the presence of rLOX-PP-ATTO565, and probed with isotype control antibody for anti-phosphorylated-H2AX; row B4, DU145 cells treated with IR in the absence of rLOX-PP-ATTO565, and probed for phosphorylated-H2AX; row B5, DU145 cells treated with IR and rLOX-PP-ATTO565, and probed with anti-phosphorylated-H2AX and visualized for presence of rLOX-PP-ATTO565; row B6, DU145 cells treated with IR and rLOX-PP-ATTO565, and probed with MRE 11- and anti-LOX-PP antibody; and row B7, DU145 cells treated with IR and rLOX-PP-ATTO565, and probed with anti-phosphorylated-H2AX and MRE 11 antibody.
Figure 7. LOX-PP inhibits clonogenic survival in response to different doses of radiation
PC3 and DU145 cells were transduced with Empty- and rLOX-PP-expressing lentiviruses and cells were grown under standard cell culture conditions and subjected to 0, 0.5,1, 2, 3, 5, 7.5 and 10 Gy of ionizing radiation. Cells were sorted to exclude dead cells and live cells were plated at 5000 or 1000 live cells in 6-well cell culture plates. Colonies were allowed to grow for 14 days, and cultures were then fixed and then stained with crystal violet. The survival fraction was calculated as described in Materials and Methods. (A) PC3-Empty or PC3-LOX-PP cells surviving fraction as a function of radiation dose; (B) respective images of cells stained with 0.5% crystal violet; (C) DU145-Empty or DU145-LOX-PP cells surviving fraction as a function of radiation dose; (D) respective images stained with 0.5% crystal violet; (n=6); * p<0.05; student’s t-test from one representative experiment of three performed with the same outcomes.
Similar articles
- Lysyl oxidase propeptide stimulates osteoblast and osteoclast differentiation and enhances PC3 and DU145 prostate cancer cell effects on bone in vivo.
Alsulaiman M, Bais MV, Trackman PC. Alsulaiman M, et al. J Cell Commun Signal. 2016 Mar;10(1):17-31. doi: 10.1007/s12079-015-0311-9. Epub 2015 Dec 1. J Cell Commun Signal. 2016. PMID: 26627907 Free PMC article. - Recombinant lysyl oxidase propeptide protein inhibits growth and promotes apoptosis of pre-existing murine breast cancer xenografts.
Bais MV, Nugent MA, Stephens DN, Sume SS, Kirsch KH, Sonenshein GE, Trackman PC. Bais MV, et al. PLoS One. 2012;7(2):e31188. doi: 10.1371/journal.pone.0031188. Epub 2012 Feb 8. PLoS One. 2012. PMID: 22363577 Free PMC article. - Determination of cell uptake pathways for tumor inhibitor lysyl oxidase propeptide.
Ozdener GB, Bais MV, Trackman PC. Ozdener GB, et al. Mol Oncol. 2016 Jan;10(1):1-23. doi: 10.1016/j.molonc.2015.07.005. Epub 2015 Aug 6. Mol Oncol. 2016. PMID: 26297052 Free PMC article. - Functions and Mechanisms of Pro-Lysyl Oxidase Processing in Cancers and Eye Pathologies with a Focus on Diabetic Retinopathy.
Trackman PC, Peymanfar Y, Roy S. Trackman PC, et al. Int J Mol Sci. 2022 May 3;23(9):5088. doi: 10.3390/ijms23095088. Int J Mol Sci. 2022. PMID: 35563478 Free PMC article. Review. - Starving cancer cells to enhances DNA damage and immunotherapy response.
Shahi A, Kidane D. Shahi A, et al. Oncotarget. 2024 Jun 20;15:392-399. doi: 10.18632/oncotarget.28595. Oncotarget. 2024. PMID: 38900609 Free PMC article. Review.
Cited by
- Lysyl oxidase mediates hypoxia-induced radioresistance in non-small cell lung cancer A549 cells.
Gong C, Gu R, Jin H, Sun Y, Li Z, Chen J, Wu G. Gong C, et al. Exp Biol Med (Maywood). 2016 Feb;241(4):387-95. doi: 10.1177/1535370215609694. Epub 2015 Oct 28. Exp Biol Med (Maywood). 2016. PMID: 26515140 Free PMC article. - Lysyl Oxidase and the Tumor Microenvironment.
Wang TH, Hsia SM, Shieh TM. Wang TH, et al. Int J Mol Sci. 2016 Dec 29;18(1):62. doi: 10.3390/ijms18010062. Int J Mol Sci. 2016. PMID: 28036074 Free PMC article. Review. - Insights into the structure and dynamics of lysyl oxidase propeptide, a flexible protein with numerous partners.
Vallet SD, Miele AE, Uciechowska-Kaczmarzyk U, Liwo A, Duclos B, Samsonov SA, Ricard-Blum S. Vallet SD, et al. Sci Rep. 2018 Aug 6;8(1):11768. doi: 10.1038/s41598-018-30190-6. Sci Rep. 2018. PMID: 30082873 Free PMC article. - Functional importance of lysyl oxidase family propeptide regions.
Trackman PC. Trackman PC. J Cell Commun Signal. 2018 Mar;12(1):45-53. doi: 10.1007/s12079-017-0424-4. Epub 2017 Oct 30. J Cell Commun Signal. 2018. PMID: 29086201 Free PMC article. Review. - Inhibition of LSD1 epigenetically attenuates oral cancer growth and metastasis.
Alsaqer SF, Tashkandi MM, Kartha VK, Yang YT, Alkheriji Y, Salama A, Varelas X, Kukuruzinska M, Monti S, Bais MV. Alsaqer SF, et al. Oncotarget. 2017 Jul 27;8(43):73372-73386. doi: 10.18632/oncotarget.19637. eCollection 2017 Sep 26. Oncotarget. 2017. PMID: 29088714 Free PMC article.
References
- Panchenko MV, Stetler-Stevenson WG, Trubetskoy OV, Gacheru SN, Kagan HM. Metalloproteinase activity secreted by fibrogenic cells in the processing of prolysyl oxidase. Potential role of procollagen C-proteinase. The Journal of biological chemistry. 1996;271(12):7113–9. - PubMed
- Uzel MI, Scott IC, Babakhanlou-Chase H, Palamakumbura AH, Pappano WN, Hong HH, et al. Multiple bone morphogenetic protein 1-related mammalian metalloproteinases process pro-lysyl oxidase at the correct physiological site and control lysyl oxidase activation in mouse embryo fibroblast cultures. The Journal of biological chemistry. 2001;276(25):22537–43. - PubMed
- Barker HE, Cox TR, Erler JT. The rationale for targeting the LOX family in cancer. Nature reviews Cancer. 2012;12(8):540–52. - PubMed
- Min C, Kirsch KH, Zhao Y, Jeay S, Palamakumbura AH, Trackman PC, et al. The tumor suppressor activity of the lysyl oxidase propeptide reverses the invasive phenotype of Her-2/neu-driven breast cancer. Cancer research. 2007;67(3):1105–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous