Zebrafish enpp1 mutants exhibit pathological mineralization, mimicking features of generalized arterial calcification of infancy (GACI) and pseudoxanthoma elasticum (PXE) - PubMed (original) (raw)
Zebrafish enpp1 mutants exhibit pathological mineralization, mimicking features of generalized arterial calcification of infancy (GACI) and pseudoxanthoma elasticum (PXE)
Alexander Apschner et al. Dis Model Mech. 2014 Jul.
Abstract
In recent years it has become clear that, mechanistically, biomineralization is a process that has to be actively inhibited as a default state. This inhibition must be released in a rigidly controlled manner in order for mineralization to occur in skeletal elements and teeth. A central aspect of this concept is the tightly controlled balance between phosphate, a constituent of the biomineral hydroxyapatite, and pyrophosphate, a physiochemical inhibitor of mineralization. Here, we provide a detailed analysis of a zebrafish mutant, dragonfish (dgf), which is mutant for ectonucleoside pyrophosphatase/phosphodiesterase 1 (Enpp1), a protein that is crucial for supplying extracellular pyrophosphate. Generalized arterial calcification of infancy (GACI) is a fatal human disease, and the majority of cases are thought to be caused by mutations in ENPP1. Furthermore, some cases of pseudoxanthoma elasticum (PXE) have recently been linked to ENPP1. Similar to humans, we show here that zebrafish enpp1 mutants can develop ectopic calcifications in a variety of soft tissues - most notably in the skin, cartilage elements, the heart, intracranial space and the notochord sheet. Using transgenic reporter lines, we demonstrate that ectopic mineralizations in these tissues occur independently of the expression of typical osteoblast or cartilage markers. Intriguingly, we detect cells expressing the osteoclast markers Trap and CathepsinK at sites of ectopic calcification at time points when osteoclasts are not yet present in wild-type siblings. Treatment with the bisphosphonate etidronate rescues aspects of the dgf phenotype, and we detected deregulated expression of genes that are involved in phosphate homeostasis and mineralization, such as fgf23, npt2a, entpd5 and spp1 (also known as osteopontin). Employing a UAS-GalFF approach, we show that forced expression of enpp1 in blood vessels or the floorplate of mutant embryos is sufficient to rescue the notochord mineralization phenotype. This indicates that enpp1 can exert its function in tissues that are remote from its site of expression.
Keywords: Ectopic mineralization; GACI; Generalized arterial calcification of infancy; PXE; Pseudoxanthoma elasticum; Pyrophosphate; Zebrafish.
© 2014. Published by The Company of Biologists Ltd.
Figures
Fig. 1.
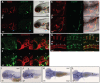
_dgf_hu4581 mutants show decreased phosphodiesterase activity and multiple ectopic calcifications. (A) Depiction of the Enpp1 protein structure; the _dgf_hu4581 allele represents a frame shift at amino acid 331, leading to a premature stop codon. (B) Phosphodiesterase I activity is significantly reduced in the lysate of dgf embryos. Means±1 s.d. are shown. Sib(s), wild-type sibling(s). Alizarin-Red (staining mineralized tissue) and Alcian-Blue (staining cartilage) staining of a sibling embryo (C) and dgf mutant (D) at 8 dpf showing extensive ectopic calcification of the notochord, as well as calcification of the neural tube (D, arrowhead). (E) Ventral view of ceratohyal cartilage element of sibling embryo; in mutant embryos, early onset of perichondral ossification (F, red arrowhead), as well as spots of ectopic cartilage calcification (F, black arrowhead), were observed. van Kossa (brown, staining mineralized tissue) and van Gieson (red, staining osteoid) staining on transverse sections of the brain of a sibling (G) and a dgf mutant with intracranial calcification (H). Transverse section through the heart region of sibling (I) and mutant (J) embryos, both displaying mineralized cleithra (cl) and basobranchial (bb). Mutants (J) in addition display ectopic mineralization between myocard and epicard (red arrowhead) and within the heart (blue arrowhead). (K) Transverse section at the level of the yolk sac of a sibling; (L) the mutant displays ectopic mineralization of the skin. Axial skeleton at the level of the dorsal fin of a sibling (M) and mutant (N) 4-week-old (4wk) fish. Mutants display not only fusion of vertebral bodies but also of neural and haemal arches (N). Alizarin-Red staining of juvenile sibling (O) and mutant (P and enlarged image of the indicated area in P′). Note the ectopic mineralization at the ethmoid plate cartilage element (green arrowhead in P) and nodules of mineralization at the dentary (black arrowheads in P,P′). (Q) Alizarin-Red staining showing ectopic mineralization (black arrowheads) surrounding the eye of a dgf adult mutant (also green arrowhead in P). (R) In the heart of adult zebrafish, no mineralization was visible in siblings. (S) In mutants extensive ectopic calcification was found upon Alizarin-Red staining in the bulbus arteriosus (black arrowhead) but not in the ventral aorta (green arrowhead). Bb, basobranchial; Cl, cleithrum; y, yolk.
Fig. 2.
Soft tissue calcifications in dgf mutants probably represent passive calcium depositions. collagen10a1:YFP (col10) transgene in sibling (A) and mutant (B). Note that no expression of collagen10a1 was detected at sites of ectopic mineralization (Alizarin Red) at the heart (B, blue arrow). osteocalcin:GFP (osc) combined with Alizarin staining in siblings (C) and dgf mutants (D), no ectopic expression of osc was observed to colocalize with ectopic mineralization in the heart region and pectoral fin (D, blue arrows). Calcein staining marks calcifications in collagen2a1a:mCherry (col2) transgenic line in wild-type siblings (E) and dgf mutants (F). The dgf mutant shows ectopic calcifications in the cranium (F, white arrows), however no ectopic expression of collagen2a1a was observed. Alizarin staining and collagen10a1:YFP transgene expression in the axial skeleton of a sibling (G) and dgf embryo (H), ectopic mineralization of the notochord sheet occurs independently of collagen10a1 expression (white arrows, H). In situ hybridization for spp1 (blue) in siblings (I,K) and dgf mutants (J,L). Note upregulation of spp1 in mutant bone elements. Further ectopic expression occurs at loci that are frequently affected by ectopic mineralization in mutants (arrowheads in J,L; compare with
supplementary material Fig. S1H
). cl, cleithrum; ps, parasphenoid; sib, sibling.
Fig. 3.
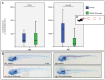
Treatment with the pyrophosphate analog etidronate rescues aspects of the dgf phenotype. (A) Measurements (in pixel area) of the mineralized area in the axial skeleton of Alizarin-Red- and Alcian-Blue-stained siblings (sibs; left panel) and mutants (right panel), which were either untreated (blue) or treated with 100 μM Etidronate (green). A′ indicates the region of interest that was measured for the analysis. No significant difference (ns) occurs in siblings (A,B left panels); in treated dgf mutants, the mineralized area was significantly reduced when compared with untreated dgf mutants (A,B right panels). _n_=50 (dgf 100 μM Etidronate); _n_=48 (dgf control); _n_= 24 (siblings 100 μM Etidronate); _n_=24 (siblings control).
Fig. 4.
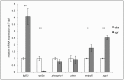
Expression of regulators of phosphate and biomineralization is perturbed in dgf mutants. qPCR analysis showing the relative gene expression levels of genes involved in phosphate homeostasis and biomineralization in siblings (sib) compared with those in dgf mutants, normalized to the expression of ef2a. *_P_≤0.05, **_P_≤0.01. Means±s.e.m. are shown.
Fig. 5.
Distal expression of enpp1 is sufficient to prevent ectopic calcifications in the notochord. (A) Overview of the tissue-specific lines that were used in the different rescue experiments. (B) Analysis and quantification of dgf embryos and dgf transgenic lines with respect to the notochord phenotype (shown as percentages of the total number of embryos examined). Sibs, siblings; transg, transgenic line. (C–C‴) Representative examples of non-transgenic dgf embryos and their transgenic dgf siblings for the respective constructs after Alizarin-Red staining.
Fig. 6.
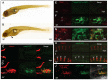
Cells expressing osteoclastic markers appear at ectopic mineralization sites in dgf mutants. Staining of Trap in a wild-type sibling (A) and dgf embryo (B). Trap staining (red) was visible in a dgf embryo at the region of the heart and yolk sac (B, arrowhead). Ventral view of sibling (C) and mutant (D) embryo with ectopic mineralization in the heart and yolk sac region. (C) No _cathepsinK_-positive cells were visible in siblings at this timepoint; (D) mutants showed colocalization of ectopic soft tissue mineralization and _cathepsinK_-positive cells, but no _cathepsinK_-positive cells were aligned to skeletal elements, such as the cleithrum (cl). (E,F) Ventral view of the yolk sac area of embryos that had been stained for Trap and with an antibody against GFP (α GFP). (F) Trap staining appeared in association with _cathepsinK_-positive cells. White arrows indicate loci of osteoclasts with high Trap activity. (G) Higher-magnification image of cathepsinK and Trap colocalization in a dgf mutant. Scale bar: 20μm. The staining of Trap in E–G is pseudo-colored for improved visibility. In the axial skeleton, _cathepsinK_-positive cells were not visible in siblings (H); however, in dgf mutants (I), _cathepsinK_-positive cells appeared from 9 dpf onwards and colocalized with mineralized vertebral bodies. White arrows indicate osteoclasts aligning with vertebral elements. (J) Ventral view of accumulating _cathepsinK_-positive cells in the skin of the heart region. These cells also express the macrophage marker mpeg1.
Similar articles
- Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6.
Nitschke Y, Baujat G, Botschen U, Wittkampf T, du Moulin M, Stella J, Le Merrer M, Guest G, Lambot K, Tazarourte-Pinturier MF, Chassaing N, Roche O, Feenstra I, Loechner K, Deshpande C, Garber SJ, Chikarmane R, Steinmann B, Shahinyan T, Martorell L, Davies J, Smith WE, Kahler SG, McCulloch M, Wraige E, Loidi L, Höhne W, Martin L, Hadj-Rabia S, Terkeltaub R, Rutsch F. Nitschke Y, et al. Am J Hum Genet. 2012 Jan 13;90(1):25-39. doi: 10.1016/j.ajhg.2011.11.020. Epub 2011 Dec 29. Am J Hum Genet. 2012. PMID: 22209248 Free PMC article. - Oral supplementation of inorganic pyrophosphate in pseudoxanthoma elasticum.
Kozák E, Fülöp K, Tőkési N, Rao N, Li Q, Terry SF, Uitto J, Zhang X, Becker C, Váradi A, Pomozi V. Kozák E, et al. Exp Dermatol. 2022 Apr;31(4):548-555. doi: 10.1111/exd.14498. Epub 2021 Nov 17. Exp Dermatol. 2022. PMID: 34758173 Free PMC article. - Paediatric pseudoxanthoma elasticum with cardiovascular involvement.
Li Q, Baker J, Kowalczyk J, Jiang Q, Uitto J, Schachner L. Li Q, et al. Br J Dermatol. 2013 Nov;169(5):1148-51. doi: 10.1111/bjd.12462. Br J Dermatol. 2013. PMID: 23746223 Free PMC article. - ABCC6, Pyrophosphate and Ectopic Calcification: Therapeutic Solutions.
Shimada BK, Pomozi V, Zoll J, Kuo S, Martin L, Le Saux O. Shimada BK, et al. Int J Mol Sci. 2021 Apr 27;22(9):4555. doi: 10.3390/ijms22094555. Int J Mol Sci. 2021. PMID: 33925341 Free PMC article. Review. - Pseudoxanthoma Elasticum as a Paradigm of Heritable Ectopic Mineralization Disorders: Pathomechanisms and Treatment Development.
Li Q, van de Wetering K, Uitto J. Li Q, et al. Am J Pathol. 2019 Feb;189(2):216-225. doi: 10.1016/j.ajpath.2018.09.014. Epub 2018 Nov 7. Am J Pathol. 2019. PMID: 30414410 Free PMC article. Review.
Cited by
- Zebrafish as a Model to Study Vascular Elastic Fibers and Associated Pathologies.
Hoareau M, El Kholti N, Debret R, Lambert E. Hoareau M, et al. Int J Mol Sci. 2022 Feb 14;23(4):2102. doi: 10.3390/ijms23042102. Int J Mol Sci. 2022. PMID: 35216218 Free PMC article. Review. - Type II Na+-phosphate Cotransporters and Phosphate Balance in Teleost Fish.
Verri T, Werner A. Verri T, et al. Pflugers Arch. 2019 Jan;471(1):193-212. doi: 10.1007/s00424-018-2239-4. Epub 2018 Dec 12. Pflugers Arch. 2019. PMID: 30542786 Review. - Zebrafish fin regeneration involves generic and regeneration-specific osteoblast injury responses.
Sehring I, Mohammadi HF, Haffner-Luntzer M, Ignatius A, Huber-Lang M, Weidinger G. Sehring I, et al. Elife. 2022 Jun 24;11:e77614. doi: 10.7554/eLife.77614. Elife. 2022. PMID: 35748539 Free PMC article. - Zebrafish Craniofacial Development: A Window into Early Patterning.
Mork L, Crump G. Mork L, et al. Curr Top Dev Biol. 2015;115:235-69. doi: 10.1016/bs.ctdb.2015.07.001. Epub 2015 Oct 6. Curr Top Dev Biol. 2015. PMID: 26589928 Free PMC article. Review. - High resolution DLP stereolithography to fabricate biocompatible hydroxyapatite structures that support osteogenesis.
Martinez JS, Peterson S, Hoel CA, Erno DJ, Murray T, Boyd L, Her JH, Mclean N, Davis R, Ginty F, Duclos SJ, Davis BM, Parthasarathy G. Martinez JS, et al. PLoS One. 2022 Aug 8;17(8):e0272283. doi: 10.1371/journal.pone.0272283. eCollection 2022. PLoS One. 2022. PMID: 35939440 Free PMC article.
References
- Aiba A., Nakajima A., Okawa A., Koda M., Yamazaki M. (2009). Evidence of enhanced expression of osteopontin in spinal hyperostosis of the twy mouse. Spine 34, 1644–1649 - PubMed
- Apschner A., Schulte-Merker S., Witten P. E. (2011). Chapter 10 – Not all bones are created equal – using zebrafish and other teleost species in osteogenesis research. In Methods in Cell Biology, Vol. 105 (ed. Westerfield M., Dietrich H. William, Zon L. I.). Waltham, MA: Academic Press - PubMed
- Bancroft J. S. A. (1996a). van Gieson Theory and Practice of Histological Techniques. pp. 127–188 New York, NY: Churchill Livingstone
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous