Rewiring of cellular membrane homeostasis by picornaviruses - PubMed (original) (raw)
Review
. 2014 Sep 1;88(17):9478-89.
doi: 10.1128/JVI.00922-14. Epub 2014 Jun 11.
Affiliations
- PMID: 24920802
- PMCID: PMC4136358
- DOI: 10.1128/JVI.00922-14
Review
Rewiring of cellular membrane homeostasis by picornaviruses
George A Belov et al. J Virol. 2014.
Abstract
Viruses are obligatory intracellular parasites and utilize host elements to support key viral processes, including penetration of the plasma membrane, initiation of infection, replication, and suppression of the host's antiviral defenses. In this review, we focus on picornaviruses, a family of positive-strand RNA viruses, and discuss the mechanisms by which these viruses hijack the cellular machinery to form and operate membranous replication complexes. Studies aimed at revealing factors required for the establishment of viral replication structures identified several cellular-membrane-remodeling proteins and led to the development of models in which the virus used a preexisting cellular-membrane-shaping pathway "as is" for generating its replication organelles. However, as more data accumulate, this view is being increasingly questioned, and it is becoming clearer that viruses may utilize cellular factors in ways that are distinct from the normal functions of these proteins in uninfected cells. In addition, the proteincentric view is being supplemented by important new studies showing a previously unappreciated deep remodeling of lipid homeostasis, including extreme changes to phospholipid biosynthesis and cholesterol trafficking. The data on viral modifications of lipid biosynthetic pathways are still rudimentary, but it appears once again that the viruses may rewire existing pathways to generate novel functions. Despite remarkable progress, our understanding of how a handful of viral proteins can completely overrun the multilayered, complex mechanisms that control the membrane organization of a eukaryotic cell remains very limited.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
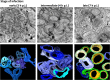
FIG 1
Virus-induced membrane remodeling in poliovirus-infected HeLa cells. EM and 3D tomographic reconstructions of membranes at early (3 h postinfection [p.i.]), intermediate (4 h p.i.), and late (7 h p.i.) time points after infection of HeLa cells with poliovirus. (Modified from reference .)
FIG 2
(A) Intracellular transport pathways. Diagram depicting the compartments of the secretory and endosomal pathways. Colors indicate the known coats: COPII (brown), COPI (green), and clathrin (red). PM, plasma membrane. Secretory cargos are synthesized in the ER, exit the ER at ERES in COPII-coated vesicles, and are transported to ERGIC. Cargos are sorted from ERGIC into anterograde carriers that move them to the Golgi compartment. After passage through the Golgi compartment, cargos are sorted at TGN for delivery to the PM and early and late endosomes. A COPI-mediated pathway recycles proteins from the Golgi compartment and ERGIC and returns them to the ER. (B) GBF1-mediated Arf activation. Formation of ER-Golgi transport vesicles requires activation of an Arf by GBF1 (step 1), recruitment of a coat (step 2), and cargo sorting/concentration (step 3) into a nascent bud. Additional proteins participate in each step but are not depicted for simplicity. (C) Distinct GBF1 function in viral replication and cellular secretion. The highly conserved domains of GBF1 include a dimerization and cyclophilin binding (DCB) domain, a homology upstream of Sec7d (HUS) domain, the catalytic Sec7d, and three homology downstream of Sec7d (HDS1-3) domains. The region of GBF1 capable of interacting with 3A of poliovirus (bar) is distinct from the Sec7d that binds the substrate Arf (bar). Truncated forms of GBF1 were expressed as a sole functional copy in HeLa cells and tested for their ability to support poliovirus replication and cellular secretion as described in reference . The removal of the N-terminal region inhibits both replication and secretion, while deletions of the C terminus have less effect on replication but inhibit secretion. (D) GBF1 interactome. Proteins known to bind mammalian GBF1 are depicted in blue. Proteins shown to bind to the yeast ortholog of GBF1, Gea1/2, are depicted in purple. Cyclophilin is predicted to bind GBF1 based on homology with a plant ortholog and is depicted in black. The 3A poliovirus protein is in green. (E) Model for possible 3A-induced alterations in the GBF1 interactome. The top diagram shows GBF1 interactions in an uninfected cell. The bottom diagram shows inhibition of a subset of GBF1 interactions in infected cells and the establishment of new interactions that are prohibited in uninfected cells, likely resulting in significant changes in the overall GBF1 interactome on the replication membranes compared to that on the membranes in uninfected cells.
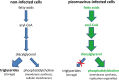
FIG 3
Virus-induced changes in lipid metabolism. Uninfected cells channel the majority of fatty acids into triglycerides to be stored in lipid droplets. In picornavirus-infected cells, the import of long-chain fatty acids is highly activated, but triglyceride synthesis is inhibited. This fuels the increased production of phosphatidylcholine to generate the membranous scaffold of the replication organelles.
Similar articles
- Dynamic lipid landscape of picornavirus replication organelles.
Belov GA. Belov GA. Curr Opin Virol. 2016 Aug;19:1-6. doi: 10.1016/j.coviro.2016.05.003. Epub 2016 May 27. Curr Opin Virol. 2016. PMID: 27240115 Free PMC article. Review. - Modulation of lipid synthesis and trafficking pathways by picornaviruses.
Belov GA. Belov GA. Curr Opin Virol. 2014 Dec;9:19-23. doi: 10.1016/j.coviro.2014.08.007. Epub 2014 Sep 18. Curr Opin Virol. 2014. PMID: 25240228 Review. - Cholesterol shuttling is important for RNA replication of coxsackievirus B3 and encephalomyocarditis virus.
Albulescu L, Wubbolts R, van Kuppeveld FJ, Strating JR. Albulescu L, et al. Cell Microbiol. 2015 Aug;17(8):1144-56. doi: 10.1111/cmi.12425. Epub 2015 Mar 10. Cell Microbiol. 2015. PMID: 25645595 - Model of OSBP-Mediated Cholesterol Supply to Aichi Virus RNA Replication Sites Involving Protein-Protein Interactions among Viral Proteins, ACBD3, OSBP, VAP-A/B, and SAC1.
Ishikawa-Sasaki K, Nagashima S, Taniguchi K, Sasaki J. Ishikawa-Sasaki K, et al. J Virol. 2018 Mar 28;92(8):e01952-17. doi: 10.1128/JVI.01952-17. Print 2018 Apr 15. J Virol. 2018. PMID: 29367253 Free PMC article. - Mechanisms of Cellular Membrane Reorganization to Support Hepatitis C Virus Replication.
Wang H, Tai AW. Wang H, et al. Viruses. 2016 May 20;8(5):142. doi: 10.3390/v8050142. Viruses. 2016. PMID: 27213428 Free PMC article. Review.
Cited by
- From the "One-Molecule, One-Target, One-Disease" Concept towards Looking for Multi-Target Therapeutics for Treating Non-Polio Enterovirus (NPEV) Infections.
Roux H, Touret F, Rathelot P, Vanelle P, Roche M. Roux H, et al. Pharmaceuticals (Basel). 2024 Sep 16;17(9):1218. doi: 10.3390/ph17091218. Pharmaceuticals (Basel). 2024. PMID: 39338380 Free PMC article. Review. - Modulation of nucleotide metabolism by picornaviruses.
Nouwen LV, Breeuwsma M, Zaal EA, van de Lest CHA, Buitendijk I, Zwaagstra M, Balić P, Filippov DV, Berkers CR, van Kuppeveld FJM. Nouwen LV, et al. PLoS Pathog. 2024 Mar 8;20(3):e1012036. doi: 10.1371/journal.ppat.1012036. eCollection 2024 Mar. PLoS Pathog. 2024. PMID: 38457376 Free PMC article. - Fine Structure of Plasmodesmata-Associated Membrane Bodies Formed by Viral Movement Protein.
Atabekova AK, Golyshev SA, Lezzhov AA, Skulachev BI, Moiseenko AV, Yastrebova DM, Andrianova NV, Solovyev ID, Savitsky AP, Morozov SY, Solovyev AG. Atabekova AK, et al. Plants (Basel). 2023 Dec 7;12(24):4100. doi: 10.3390/plants12244100. Plants (Basel). 2023. PMID: 38140427 Free PMC article. - Curcumol inhibits EMCV replication by activating CH25H and inhibiting the formation of ROs.
Zheng J, Sun P, Sun N, Hao Z, Fan K, Yin W, Khan A, Guo J, Zheng X, Li H. Zheng J, et al. BMC Vet Res. 2022 Dec 26;18(1):453. doi: 10.1186/s12917-022-03531-x. BMC Vet Res. 2022. PMID: 36572890 Free PMC article. - Foot-and-Mouth Disease Virus 3A Hijacks Sar1 and Sec12 for ER Remodeling in a COPII-Independent Manner.
Lee HW, Jiang YF, Chang HW, Cheng IC. Lee HW, et al. Viruses. 2022 Apr 18;14(4):839. doi: 10.3390/v14040839. Viruses. 2022. PMID: 35458569 Free PMC article.
References
- Ehrenfeld E, Domingo E, Roos RP. (ed). 2010. The picornaviruses. ASM Press, Washington, DC
- Nchoutmboube JA, Viktorova EG, Scott AJ, Ford LA, Pei Z, Watkins PA, Ernst RK, Belov GA. 2013. Increased long chain acyl-Coa synthetase activity and fatty acid import is linked to membrane synthesis for development of picornavirus replication organelles. PLoS Pathog. 9:e1003401. 10.1371/journal.ppat.1003401 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources