Genistein inhibits osteoclastic differentiation of RAW 264.7 cells via regulation of ROS production and scavenging - PubMed (original) (raw)
Genistein inhibits osteoclastic differentiation of RAW 264.7 cells via regulation of ROS production and scavenging
Sang-Hyun Lee et al. Int J Mol Sci. 2014.
Abstract
Genistein, a phytoestrogen, has been demonstrated to have a bone-sparing and antiresorptive effect. Genistein can inhibit the osteoclast formation of receptor activator of nuclear factor-κB ligand (RANKL)-induced RAW 264.7 cells by preventing the translocation of nuclear factor-κB (NF-κB), a redox-sensitive factor, to the nucleus. Therefore, the suppressive effect of genistein on the reactive oxygen species (ROS) level during osteoclast differentiation and the mechanism associated with the control of ROS levels by genistein were investigated. The cellular antioxidant capacity and inhibitory effect of genistein were confirmed. The translation and activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 1 (Nox1), as well as the disruption of the mitochondrial electron transport chain system were obviously suppressed by genistein in a dose-dependent manner. The induction of phase II antioxidant enzymes, such as superoxide dismutase 1 (SOD1) and heme oxygenase-1 (HO-1), was enhanced by genistein. In addition, the translational induction of nuclear factor erythroid 2-related factor 2 (Nrf2) was notably increased by genistein. These results provide that the inhibitory effects of genistein on RANKL-stimulated osteoclast differentiation is likely to be attributed to the control of ROS generation through suppressing the translation and activation of Nox1 and the disruption of the mitochondrial electron transport chain system, as well as ROS scavenging through the Nrf2-mediated induction of phase II antioxidant enzymes, such as SOD1 and HO-1.
Figures
Figure 1
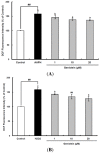
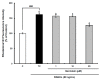
Cellular antioxidant capacity of genistein against oxidative stress induced by 2,2'-azobis(2-amidinopropane) dihydrochloride (AAPH) (A) and H2O2 (B) in HepG2 cells. HepG2 cells were first cultured in 96-well plates (5 × 105/mL) with Dulbecco’s modified Eagle’s medium (DMEM) for 24 h. After the cells were incubated with different concentrations of sample dissolved in dimethylsulfoxide (DMSO) for 30 min, Hank’s balanced salt solution (HBSS), which is fluorescently stable, was then added to each well. After the cells were treated with 60 μM AAPH or 1 mM H2O2 for 30 min, 2',7'-dichlorodihydrofluorescein-diacetate (DCFH-DA) was added to the culture plates at a final concentration of 40 µM, and the cells were incubated for 30 min at 37 °C in the dark. Then, the cells were washed with HBSS and 2',7'-dichlorofluorescein (DCF) fluorescence intensity was measured at an excitation wavelength of 485 nm and an emission wavelength of 535 nm using a Tecan GENios fluorometric plate reader. Data are expressed as percentages of the value of untreated cells (mean ± standard deviation, n = 3). Different corresponding letters indicate significant differences at p < 0.05 by Duncan’s test. ## p < 0.01 vs. control.
Figure 2
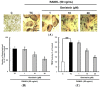
Inhibitory effects of genistein on differentiation (A,B) and tartrate-resistant acid phosphatase (TRAP) activity (C) of osteoclastic RAW 264.7 cells. RAW 264.7 cells were exposed to the receptor activator of nuclear factor kappa-B ligand (RANKL) (50 ng/mL) for five days in the absence and the presence of genistein. After five days in culture, the cells were fixed and stained using a leukocyte acid phosphatase kit. TRAP-positive multinucleated osteoclasts were visualized under light microphotography (A); TRAP-positive multi-nucleated osteoclasts were counted (B). Data are expressed as the percentages of the value of cells treated with RANKL (means ± standard deviations (SD), n = 3); TRAP activity was measured at λ = 405 nm (C). Data are expressed as percentages of the values of untreated cells (means ± standard deviations, n = 3). Different corresponding letters indicate significant differences at p < 0.05 by Duncan’s test. ### p < 0.001 vs. C (C: control, which was not treated; TC: treated control, which was treated with RANKL).
Figure 3
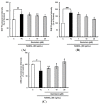
The suppressive effects of genistein on general ROS (A), superoxide anions (B) and GSH levels (C) during the receptor activator of nuclear factor kappa-B ligand (RANKL)-induced osteoclast differentiation. RAW 264.7 cells were seeded in 12-well plates (3 × 104 cells/well) containing DMEM medium plus 10% FBS and incubated for 24 h. The medium was then replaced with a differentiation medium containing 50 ng/mL RANKL, and genistein at 1–10 µM was tested. After two days of incubation, the wells were gently washed twice with PBS. HBSS, which is fluorescently stable, was then added to each well instead of normal medium. DCFH-DA, dihydroethidium (DHE) or monochlorobimane (mBCl) was added to the culture plates at a final concentration of 40, 50 or 50 μM, respectively, and incubated for 30 min at 37 °C in the dark. After the cells were washed twice with HBSS, the fluorescence intensity of 2',7'-dichlorofluorescein (DCF), DHE and mBCl were measured using a Tecan GENios fluorometric plate reader. Data are expressed as the percentages of the value of untreated cells (means ± standard deviations, n = 3). Different corresponding letters indicate significant differences. Different corresponding letters indicate significant differences at p < 0.05 by Duncan’s test. # p < 0.05, ## p < 0.01, ### p < 0.001 vs. C (C: control, which was not treated; TC: treated control, which was treated with RANKL).
Figure 4
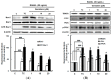
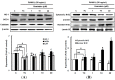
The effect of genistein on the translation and activation of NADPH oxidase (Nox1) (A) and its upstream signals (B). RAW 264.7 cells were exposed to the receptor activator of nuclear factor kappa-B ligand (RANKL) (50 ng/mL) for two days in the absence and presence of genistein. GTP-Rac1 was recovered by centrifugation from proteins complexed to the beads, which have a glutathione _S_-transferase-p21-binding domain: human Pak1 as 67–150 and binds to GTP-bound Rac1 of the lysates. Western blotting analysis was performed on the lysates of cells that had been incubated with 1–20 μm genistein. Different corresponding letters indicate significant differences at p < 0.05 by Duncan’s test. ## p < 0.01, ### p < 0.001 vs. C (C: control, which was not treated; TC: treated control, which was treated with RANKL).
Figure 5
The effect of genistein on mitochondrial membrane potential in RANKL-treated Raw264.7 cells. RAW 264.7 cells were exposed to the receptor activator of nuclear factor kappa-B ligand (RANKL) (50 ng/mL) for two days in the absence and the presence of genistein. After two days of incubation, the wells were gently washed twice with PBS. HBSS, which is fluorescently stable, was then added to each well. Rhodamine 123, a fluorescent probe detecting mitochondrial membrane potential, was added to the culture plates at a final concentration of 5 µM and incubated for 30 min at 37 °C in the dark. After the cells were washed with HBSS, the fluorescence intensity of rhodamine 123 was measured with an excitation wavelength of 485 nm and an emission wavelength of 535 nm using a Tecan GENios fluorometric plate reader. Data are expressed as percentages of the values of untreated cells (means ± standard deviations, n = 3). Different corresponding letters indicate significant differences at p < 0.05 by Duncan’s test. ### p < 0.001 vs. C (C: control, which was not treated; TC: treated control, which was treated with RANKL).
Figure 6
The effects of genistein on the expression of detoxifying and antioxidant enzymes (A) and the activation of Nrf2 (B) in RANKL-treated RAW 264.7 cells. RAW 264.7 cells were exposed to the receptor activator of nuclear factor kappa-B ligand (RANKL) (50 ng/mL) for two days in the absence and presence of genistein. RAW 264.7 cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl (pH 8.0), 1% nonyl phenoxypolyethoxylethanol (NP)-40, 0.5% sodium deoxycholate, 150 mM NaCl and 1 mM phenylmethylsulfonyl fluoride (PMSF)) that contained a phosphatase inhibitor cocktail. The nuclear proteins in the RAW 264.7 cells were extracted by using Buffer A (10 mM hydroxyethyl piperazineethanesulfonic acid (HEPES), pH 7.9), 10 mM KCl, 2 mM MgCl2, 1 mM dithiothreitol (DTT), 0.5 mM ethylenediamine tetra-acetic acid (EDTA) (pH 7.9), 0.1 μM PMSF and 1× protease inhibitor cocktail) and Buffer B (50 mM HEPES (pH 7.9), 50 mM KCl, 0.3 mM NaCl, 1 mm DTT, 1 mM EDTA (pH 7.9), 0.1 μM PMSF and 10% glycerol). Western blotting analysis was performed on the lysates of cells that had been incubated with 1–20 μm genistein. Different corresponding letters indicate significant differences at p < 0.05 by Duncan’s test. # p < 0.05, ### p < 0.001 vs. C (C: control, which was not treated; TC: treated control, which was treated with RANKL; NS: not significant).
Similar articles
- Scoparone attenuates RANKL-induced osteoclastic differentiation through controlling reactive oxygen species production and scavenging.
Lee SH, Jang HD. Lee SH, et al. Exp Cell Res. 2015 Feb 15;331(2):267-77. doi: 10.1016/j.yexcr.2014.12.018. Epub 2015 Jan 6. Exp Cell Res. 2015. PMID: 25576385 - Nrf2 deficiency induces oxidative stress and promotes RANKL-induced osteoclast differentiation.
Hyeon S, Lee H, Yang Y, Jeong W. Hyeon S, et al. Free Radic Biol Med. 2013 Dec;65:789-799. doi: 10.1016/j.freeradbiomed.2013.08.005. Epub 2013 Aug 14. Free Radic Biol Med. 2013. PMID: 23954472 - Fisetin inhibits osteoclastogenesis through prevention of RANKL-induced ROS production by Nrf2-mediated up-regulation of phase II antioxidant enzymes.
Sakai E, Shimada-Sugawara M, Yamaguchi Y, Sakamoto H, Fumimoto R, Fukuma Y, Nishishita K, Okamoto K, Tsukuba T. Sakai E, et al. J Pharmacol Sci. 2013;121(4):288-98. doi: 10.1254/jphs.12243fp. Epub 2013 Mar 29. J Pharmacol Sci. 2013. PMID: 23538677 - Carbon monoxide - beyond toxicity?
Stucki D, Stahl W. Stucki D, et al. Toxicol Lett. 2020 Oct 15;333:251-260. doi: 10.1016/j.toxlet.2020.08.010. Epub 2020 Aug 26. Toxicol Lett. 2020. PMID: 32860873 Review. - Unraveling the Role of Reactive Oxygen Species in T Lymphocyte Signaling.
Gülow K, Tümen D, Heumann P, Schmid S, Kandulski A, Müller M, Kunst C. Gülow K, et al. Int J Mol Sci. 2024 Jun 1;25(11):6114. doi: 10.3390/ijms25116114. Int J Mol Sci. 2024. PMID: 38892300 Free PMC article. Review.
Cited by
- Alliin Attenuated RANKL-Induced Osteoclastogenesis by Scavenging Reactive Oxygen Species through Inhibiting Nox1.
Chen Y, Sun J, Dou C, Li N, Kang F, Wang Y, Cao Z, Yang X, Dong S. Chen Y, et al. Int J Mol Sci. 2016 Sep 20;17(9):1516. doi: 10.3390/ijms17091516. Int J Mol Sci. 2016. PMID: 27657047 Free PMC article. - Reactive Oxygen Species in Osteoclast Differentiation and Possible Pharmaceutical Targets of ROS-Mediated Osteoclast Diseases.
Agidigbi TS, Kim C. Agidigbi TS, et al. Int J Mol Sci. 2019 Jul 22;20(14):3576. doi: 10.3390/ijms20143576. Int J Mol Sci. 2019. PMID: 31336616 Free PMC article. Review. - Theaflavin-3, 3'-Digallate Suppresses RANKL-Induced Osteoclastogenesis and Attenuates Ovariectomy-Induced Bone Loss in Mice.
Ai Z, Wu Y, Yu M, Li J, Li S. Ai Z, et al. Front Pharmacol. 2020 Jun 29;11:803. doi: 10.3389/fphar.2020.00803. eCollection 2020. Front Pharmacol. 2020. PMID: 32694992 Free PMC article. - BNTA alleviates inflammatory osteolysis by the SOD mediated anti-oxidation and anti-inflammation effect on inhibiting osteoclastogenesis.
Wang H, Cao X, Guo J, Yang X, Sun X, Fu Z, Qin A, Wu Y, Zhao J. Wang H, et al. Front Pharmacol. 2022 Sep 29;13:939929. doi: 10.3389/fphar.2022.939929. eCollection 2022. Front Pharmacol. 2022. PMID: 36249770 Free PMC article. - Biological evaluation of isoflavonoids from Genista halacsyi using estrogen-target cells: Activities of glucosides compared to aglycones.
Fokialakis N, Alexi X, Aligiannis N, Boulaka A, Meligova AK, Lambrinidis G, Kalpoutzakis E, Pratsinis H, Cheilari A, Mitsiou DJ, Mitakou S, Alexis MN. Fokialakis N, et al. PLoS One. 2019 Jan 8;14(1):e0210247. doi: 10.1371/journal.pone.0210247. eCollection 2019. PLoS One. 2019. PMID: 30620769 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous