Correcting direct effects of ethanol on translation and transcription machinery confers ethanol tolerance in bacteria - PubMed (original) (raw)
. 2014 Jun 24;111(25):E2576-85.
doi: 10.1073/pnas.1401853111. Epub 2014 Jun 9.
David H Keating 1, Tyler Schwaegler 1, Michael S Schwalbach 1, Jeffrey Vinokur 1, Mary Tremaine 1, Jason M Peters 2, Matthew V Kotlajich 3, Edward L Pohlmann 1, Irene M Ong 1, Jeffrey A Grass 1, Patricia J Kiley 4, Robert Landick 5
Affiliations
- PMID: 24927582
- PMCID: PMC4078849
- DOI: 10.1073/pnas.1401853111
Correcting direct effects of ethanol on translation and transcription machinery confers ethanol tolerance in bacteria
Rembrandt J F Haft et al. Proc Natl Acad Sci U S A. 2014.
Abstract
The molecular mechanisms of ethanol toxicity and tolerance in bacteria, although important for biotechnology and bioenergy applications, remain incompletely understood. Genetic studies have identified potential cellular targets for ethanol and have revealed multiple mechanisms of tolerance, but it remains difficult to separate the direct and indirect effects of ethanol. We used adaptive evolution to generate spontaneous ethanol-tolerant strains of Escherichia coli, and then characterized mechanisms of toxicity and resistance using genome-scale DNAseq, RNAseq, and ribosome profiling coupled with specific assays of ribosome and RNA polymerase function. Evolved alleles of metJ, rho, and rpsQ recapitulated most of the observed ethanol tolerance, implicating translation and transcription as key processes affected by ethanol. Ethanol induced miscoding errors during protein synthesis, from which the evolved rpsQ allele protected cells by increasing ribosome accuracy. Ribosome profiling and RNAseq analyses established that ethanol negatively affects transcriptional and translational processivity. Ethanol-stressed cells exhibited ribosomal stalling at internal AUG codons, which may be ameliorated by the adaptive inactivation of the MetJ repressor of methionine biosynthesis genes. Ethanol also caused aberrant intragenic transcription termination for mRNAs with low ribosome density, which was reduced in a strain with the adaptive rho mutation. Furthermore, ethanol inhibited transcript elongation by RNA polymerase in vitro. We propose that ethanol-induced inhibition and uncoupling of mRNA and protein synthesis through direct effects on ribosomes and RNA polymerase conformations are major contributors to ethanol toxicity in E. coli, and that adaptive mutations in metJ, rho, and rpsQ help protect these central dogma processes in the presence of ethanol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
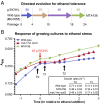
Fig. 1.
Selection of mutations conferring ethanol tolerance. (A) Summary of directed evolution experiment, with number of serial passages at each step of increasing ethanol concentration indicated. (B) Representative growth curves showing response of MG1655, MTA156, and ethanol-tolerant triple mutant (EP61) to ethanol addition. Mean growth rates for pre- and poststress cultures are shown ± SEM for four biological replicates. Time points noted were used for sampling in ribosome-profiling experiments. Wild-type MG1655 and the ethanol-tolerant triple mutant EP61 were sampled before ethanol addition (T0), during acute stress (T1), and during chronic stress (T2).
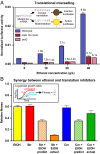
Fig. 2.
Ethanol induces toxic translational error. All panels show mean values and SEM for at least three biological replicates. (A) Normalized K529N firefly luciferase activity for wild-type (MG1655), ethanol-tolerant triple mutant (EP61), and _rpsQ_[H31P] (EP49) in the presence of indicated ethanol concentrations. Activity for the K529N mutant requires misincorporation of lysine at an AAU codon. Numbers above bars show fold increase in error relative to no-ethanol condition for the same strain. (B) Effects of treatment of wild-type MG1655 with ethanol (40 g/L) and translational inhibitors (1.6 µg Str/mL, 2 µg Cm/mL) singly and in combination. For combinatorial stresses, predicted fitness values based on a no-synergy model are shown.
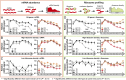
Fig. 3.
Ribosome profiling reveals ethanol-induced ribosome halting and Rho activity. (Top) A summary of ribosome profiling sample treatment for side-by-side RNAseq and ribosome footprinting, adapted from Ingolia et al. (44). (A–C) Relative mRNA signal levels from 5′ to 3′ for groups of ORFs: “all genes” (the 3,048 genes represented in mRNA and ribosome footprint datasets), high ribosome density quintile (610 genes), and low ribosome density quintile (610 genes). (D –F) Relative ribosome occupancy signal levels (ribosome footprint signal divided by mRNA signal) from 5′ to 3′ for groups of ORFs as in A–C. Data from wild-type (MG1655) cultures are shown in gray squares, and data from the ethanol-tolerant triple mutant (EP61) cultures are shown in colored triangles. Error bars show SEM. T0, T1, and T2 represent prestress, acute stress, and chronic stress conditions (Fig. 1_B_).
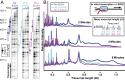
Fig. 4.
Ethanol slows transcription in vitro. (A) Representative gel lanes showing in vitro transcript elongation in the presence of 0 g, 30 g, or 60 g ethanol/L. Elongation complexes (10 nM) were halted at the end of a 26-nt C-less cassette and then exposed to ethanol. NTPs (30 μM each) were added subsequently, and products from 2-min, 4-min, and 8-min extensions were resolved by gel electrophoresis. RO indicates template run-off products. Dots to the left of the gel panels indicate the mean transcript length of RNA products for the experiment shown, and vertical lines show regions containing 15% of the signal upstream and downstream from the mean. (B) Densitometric traces of gel lanes in A. Height of traces corresponds to relative densitometric signal at a given nucleotide position. Mean average transcript lengths from two independent experiments are shown.
Fig. 5.
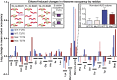
Ethanol-induced changes in ribosome occupancy by encoded amino acid. Log2 values of mean ethanol-induced ribosome occupancy changes for codons encoding the indicated amino acid are shown. Changes were measured as the ratio of poststress occupancy to prestress occupancy. Data from wild-type (MG1655) cultures are shown in blue, and data from the ethanol-tolerant triple mutant (EP61) cultures are shown in red. The Inset at upper right shows genome-wide mean ribosome occupancy values at nonstart AUG codons + SEM for T0 and T2.
Fig. 6.
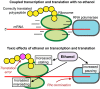
Summary of ethanol effects on transcription and translation. In unstressed cells, transcription and translation are coupled by RNAP pausing relieved by ribosome translocation (Upper). Ethanol exerts direct effects on ribosomes and RNAP that increase RNAP pausing required for Rho termination and uncouple transcription and translation via effects on the ribosome decoding center that allow Rho access to RNAP and decrease translational accuracy (Lower).
Similar articles
- Maintenance of Transcription-Translation Coupling by Elongation Factor P.
Elgamal S, Artsimovitch I, Ibba M. Elgamal S, et al. mBio. 2016 Sep 13;7(5):e01373-16. doi: 10.1128/mBio.01373-16. mBio. 2016. PMID: 27624127 Free PMC article. - Global analysis of translation termination in E. coli.
Baggett NE, Zhang Y, Gross CA. Baggett NE, et al. PLoS Genet. 2017 Mar 16;13(3):e1006676. doi: 10.1371/journal.pgen.1006676. eCollection 2017 Mar. PLoS Genet. 2017. PMID: 28301469 Free PMC article. - Transcriptional Potential Determines the Adaptability of Escherichia coli Strains with Different Fitness Backgrounds.
Kim K, Kwon SK, Kim P, Kim JF. Kim K, et al. Microbiol Spectr. 2022 Dec 21;10(6):e0252822. doi: 10.1128/spectrum.02528-22. Epub 2022 Nov 29. Microbiol Spectr. 2022. PMID: 36445144 Free PMC article. - Two Old Dogs, One New Trick: A Review of RNA Polymerase and Ribosome Interactions during Transcription-Translation Coupling.
Conn AB, Diggs S, Tam TK, Blaha GM. Conn AB, et al. Int J Mol Sci. 2019 May 27;20(10):2595. doi: 10.3390/ijms20102595. Int J Mol Sci. 2019. PMID: 31137816 Free PMC article. Review. - Dual functions of ribosome recycling factor in protein biosynthesis: disassembling the termination complex and preventing translational errors.
Janosi L, Ricker R, Kaji A. Janosi L, et al. Biochimie. 1996;78(11-12):959-69. doi: 10.1016/s0300-9084(97)86718-1. Biochimie. 1996. PMID: 9150873 Review.
Cited by
- Simultaneous achievement of high ethanol yield and titer in Clostridium thermocellum.
Tian L, Papanek B, Olson DG, Rydzak T, Holwerda EK, Zheng T, Zhou J, Maloney M, Jiang N, Giannone RJ, Hettich RL, Guss AM, Lynd LR. Tian L, et al. Biotechnol Biofuels. 2016 Jun 2;9:116. doi: 10.1186/s13068-016-0528-8. eCollection 2016. Biotechnol Biofuels. 2016. PMID: 27257435 Free PMC article. - Zmo0994, a novel LEA-like protein from Zymomonas mobilis, increases multi-abiotic stress tolerance in Escherichia coli.
Yang J, Kim HE, Jung YH, Kim J, Kim DH, Walmsley AR, Kim KH. Yang J, et al. Biotechnol Biofuels. 2020 Aug 26;13:151. doi: 10.1186/s13068-020-01790-0. eCollection 2020. Biotechnol Biofuels. 2020. PMID: 32863881 Free PMC article. - Ethanol- and PARP-Mediated Regulation of Ribosome-Associated Long Non-Coding RNA (lncRNA) in Pyramidal Neurons.
Rizavi HS, Gavin HE, Krishnan HR, Gavin DP, Sharma RP. Rizavi HS, et al. Noncoding RNA. 2023 Nov 17;9(6):72. doi: 10.3390/ncrna9060072. Noncoding RNA. 2023. PMID: 37987368 Free PMC article. - Transcriptomic and proteomic changes from medium supplementation and strain evolution in high-yielding Clostridium thermocellum strains.
Papanek B, O'Dell KB, Manga P, Giannone RJ, Klingeman DM, Hettich RL, Brown SD, Guss AM. Papanek B, et al. J Ind Microbiol Biotechnol. 2018 Nov;45(11):1007-1015. doi: 10.1007/s10295-018-2073-x. Epub 2018 Sep 5. J Ind Microbiol Biotechnol. 2018. PMID: 30187243 - Hand sanitizers as a preventive measure in COVID-19 pandemic, its characteristics, and harmful effects: a review.
Prajapati P, Desai H, Chandarana C. Prajapati P, et al. J Egypt Public Health Assoc. 2022 Feb 8;97(1):6. doi: 10.1186/s42506-021-00094-x. J Egypt Public Health Assoc. 2022. PMID: 35133535 Free PMC article. Review.
References
- Yomano LP, York SW, Ingram LO. Isolation and characterization of ethanol-tolerant mutants of Escherichia coli KO11 for fuel ethanol production. J Ind Microbiol Biotechnol. 1998;20(2):132–138. - PubMed
- Dien BS, Cotta MA, Jeffries TW. Bacteria engineered for fuel ethanol production: Current status. Appl Microbiol Biotechnol. 2003;63(3):258–266. - PubMed
- Zhao XQ, Bai FW. Mechanisms of yeast stress tolerance and its manipulation for efficient fuel ethanol production. J Biotechnol. 2009;144(1):23–30. - PubMed
- Alper H, Moxley J, Nevoigt E, Fink GR, Stephanopoulos G. Engineering yeast transcription machinery for improved ethanol tolerance and production. Science. 2006;314(5805):1565–1568. - PubMed
- Fischer CR, Klein-Marcuschamer D, Stephanopoulos G. Selection and optimization of microbial hosts for biofuels production. Metab Eng. 2008;10(6):295–304. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases