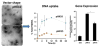Insight into nanoparticle cellular uptake and intracellular targeting - PubMed (original) (raw)
Review
Insight into nanoparticle cellular uptake and intracellular targeting
Basit Yameen et al. J Control Release. 2014.
Abstract
Collaborative efforts from the fields of biology, materials science, and engineering are leading to exciting progress in the development of nanomedicines. Since the targets of many therapeutic agents are localized in subcellular compartments, modulation of nanoparticle-cell interactions for efficient cellular uptake through the plasma membrane and the development of nanomedicines for precise delivery to subcellular compartments remain formidable challenges. Cellular internalization routes determine the post-internalization fate and intracellular localization of nanoparticles. This review highlights the cellular uptake routes most relevant to the field of non-targeted nanomedicine and presents an account of ligand-targeted nanoparticles for receptor-mediated cellular internalization as a strategy for modulating the cellular uptake of nanoparticles. Ligand-targeted nanoparticles have been the main impetus behind the progress of nanomedicines towards the clinic. This strategy has already resulted in remarkable progress towards effective oral delivery of nanomedicines that can overcome the intestinal epithelial barrier. A detailed overview of the recent developments in subcellular targeting as a novel platform for next-generation organelle-specific nanomedicines is also provided. Each section of the review includes prospects, potential, and concrete expectations from the field of targeted nanomedicines and strategies to meet those expectations.
Keywords: Intracellular distribution; Nanomedicine; Nanoparticle cellular uptake; Non-targeted and targeted nanoparticles; Subcellular targeting.
Copyright © 2014 Elsevier B.V. All rights reserved.
Conflict of interest statement
The rest of the authors declare no conflict of interest.
Figures
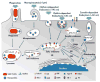
Fig. 1
Illustration of internalization pathways discussed in this article (phagocytosis, macropinocytosis, clathrin-dependent endocytosis, clathrin-independent endocytosis, and caveolin-dependent endocytosis). The fate of internalized cargo and localization to subcellular compartments are also depicted. ER: endoplasmic reticulum, NLS: nuclear localization signal, NPC: nuclear pore complex, TPP: triphenylphosphonium cation. Adapted and reproduced with permission from [90,92].
Fig. 2
(A) Schematic illustration of PSMA targeted BIND-014 docetaxel loaded polymeric nanoparticles. (B) Depiction of achievable nanomedicine attribtutes, red line represents the optimized properties. (C) CT scan evidencing a remarkable regression of lung metastases in 51 year male patient treated with two cycles of BIND-014. Reproduced with permission from [18].
Fig. 3
FcRn targeted nanoparticles can be seen as red puncta in the confocal fluorescence images of sections of mouse duodenum (left panel). Comparison of organ localization of FcRn targeted and non-targeted PLA-PEG nanoparticles (right panel). Reproduced with permission from [136].
Fig. 4
(A) Synthesis of TPP end capped and QD functionalized PLGA-PEG block copolymers. (B) Schematic illustration of drug loaded targeted nanoparticles. (C) Modulation of size and zeta potential. (D) TEM images of the fabricated nanoparticles. (E) IL-6 and TNF-α secretion profiles of nanoparticles with different zeta potentials (left) and diameters (right). Reproduced with permission from [196].
Fig. 5
TEM images revealing the effect of the number of L-lysine units on the vector shape (left). A comparison of DNA uptake capacity of pHK10 and pHK15 (middle) and gene expression (right). Reprinted with permission from [198]. Copyright (2013) American Chemical Society.
Fig. 6
(A) Scanning electron microscopic images of cytoplasmic (upper) and nucleoplasmic sides of nuclear envelope revealing the NPCs. (B) Schematic depiction of receptor mediated transportation across the NPCs. (C) Immunoelectron microscopic image of gold nanoparticles translocation between nucleus (n) and cytoplasm (c). Reproduced with permission from [207].
Similar articles
- Controlling and Monitoring Intracellular Delivery of Anticancer Polymer Nanomedicines.
Battistella C, Klok HA. Battistella C, et al. Macromol Biosci. 2017 Oct;17(10). doi: 10.1002/mabi.201700022. Epub 2017 Apr 26. Macromol Biosci. 2017. PMID: 28444959 Review. - Ligand-targeted particulate nanomedicines undergoing clinical evaluation: current status.
van der Meel R, Vehmeijer LJ, Kok RJ, Storm G, van Gaal EV. van der Meel R, et al. Adv Drug Deliv Rev. 2013 Oct;65(10):1284-98. doi: 10.1016/j.addr.2013.08.012. Epub 2013 Sep 6. Adv Drug Deliv Rev. 2013. PMID: 24018362 Review. - Advancing Nanomedicine Through Electron Microscopy: Insights Into Nanoparticle Cellular Interactions and Biomedical Applications.
Akhtar S, Zuhair F. Akhtar S, et al. Int J Nanomedicine. 2025 Mar 8;20:2847-2878. doi: 10.2147/IJN.S500978. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40078651 Free PMC article. Review. - Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine.
Donahue ND, Acar H, Wilhelm S. Donahue ND, et al. Adv Drug Deliv Rev. 2019 Mar 15;143:68-96. doi: 10.1016/j.addr.2019.04.008. Epub 2019 Apr 22. Adv Drug Deliv Rev. 2019. PMID: 31022434 Review. - Nanoparticles for Targeted Brain Drug Delivery: What Do We Know?
Pinheiro RGR, Coutinho AJ, Pinheiro M, Neves AR. Pinheiro RGR, et al. Int J Mol Sci. 2021 Oct 28;22(21):11654. doi: 10.3390/ijms222111654. Int J Mol Sci. 2021. PMID: 34769082 Free PMC article. Review.
Cited by
- Superparamagnetic Iron Oxide Nanoparticles Carrying Chemotherapeutics Improve Drug Efficacy in Monolayer and Spheroid Cell Culture by Enabling Active Accumulation.
Nguyen K, Nuß B, Mühlberger M, Unterweger H, Friedrich RP, Alexiou C, Janko C. Nguyen K, et al. Nanomaterials (Basel). 2020 Aug 11;10(8):1577. doi: 10.3390/nano10081577. Nanomaterials (Basel). 2020. PMID: 32796757 Free PMC article. - Delivery Systems for Nucleic Acids and Proteins: Barriers, Cell Capture Pathways and Nanocarriers.
Torres-Vanegas JD, Cruz JC, Reyes LH. Torres-Vanegas JD, et al. Pharmaceutics. 2021 Mar 22;13(3):428. doi: 10.3390/pharmaceutics13030428. Pharmaceutics. 2021. PMID: 33809969 Free PMC article. Review. - Functional Polymer Nanocarriers for Photodynamic Therapy.
Li T, Yan L. Li T, et al. Pharmaceuticals (Basel). 2018 Nov 30;11(4):133. doi: 10.3390/ph11040133. Pharmaceuticals (Basel). 2018. PMID: 30513613 Free PMC article. Review. - Subcellular Performance of Nanoparticles in Cancer Therapy.
Liu CG, Han YH, Kankala RK, Wang SB, Chen AZ. Liu CG, et al. Int J Nanomedicine. 2020 Feb 5;15:675-704. doi: 10.2147/IJN.S226186. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32103936 Free PMC article. Review. - Tumor-targeting delivery of herb-based drugs with cell-penetrating/tumor-targeting peptide-modified nanocarriers.
Kebebe D, Liu Y, Wu Y, Vilakhamxay M, Liu Z, Li J. Kebebe D, et al. Int J Nanomedicine. 2018 Mar 9;13:1425-1442. doi: 10.2147/IJN.S156616. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29563797 Free PMC article. Review.
References
- Feynman RP. There’s plenty of room at the bottom. Eng Sci (CalTech) 1960;23:22–36.
- Strebhardt K, Ullrich A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8:473–480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHSN268201000045C/HL/NHLBI NIH HHS/United States
- U54 CA151884/CA/NCI NIH HHS/United States
- R00CA160350/CA/NCI NIH HHS/United States
- R01 EB015419/EB/NIBIB NIH HHS/United States
- R01 EB015419-01/EB/NIBIB NIH HHS/United States
- R00 CA160350/CA/NCI NIH HHS/United States
- U54-CA151884/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical