Parallel in-register intermolecular β-sheet architectures for prion-seeded prion protein (PrP) amyloids - PubMed (original) (raw)
Parallel in-register intermolecular β-sheet architectures for prion-seeded prion protein (PrP) amyloids
Bradley R Groveman et al. J Biol Chem. 2014.
Abstract
Structures of the infectious form of prion protein (e.g. PrP(Sc) or PrP-Scrapie) remain poorly defined. The prevalent structural models of PrP(Sc) retain most of the native α-helices of the normal, noninfectious prion protein, cellular prion protein (PrP(C)), but evidence is accumulating that these helices are absent in PrP(Sc) amyloid. Moreover, recombinant PrP(C) can form amyloid fibrils in vitro that have parallel in-register intermolecular β-sheet architectures in the domains originally occupied by helices 2 and 3. Here, we provide solid-state NMR evidence that the latter is also true of initially prion-seeded recombinant PrP amyloids formed in the absence of denaturants. These results, in the context of a primarily β-sheet structure, led us to build detailed models of PrP amyloid based on parallel in-register architectures, fibrillar shapes and dimensions, and other available experimentally derived conformational constraints. Molecular dynamics simulations of PrP(90-231) octameric segments suggested that such linear fibrils, which are consistent with many features of PrP(Sc) fibrils, can have stable parallel in-register β-sheet cores. These simulations revealed that the C-terminal residues ∼124-227 more readily adopt stable tightly packed structures than the N-terminal residues ∼90-123 in the absence of cofactors. Variations in the placement of turns and loops that link the β-sheets could give rise to distinct prion strains capable of faithful template-driven propagation. Moreover, our modeling suggests that single PrP monomers can comprise the entire cross-section of fibrils that have previously been assumed to be pairs of laterally associated protofilaments. Together, these insights provide a new basis for deciphering mammalian prion structures.
Keywords: Amyloid; Molecular Dynamics; Nuclear Magnetic Resonance (NMR); Prion; Protein Structure.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
FIGURE 1.
Characterization of prion-seeded [1-13C]Ile rPrP amyloid. A, purity of the [1-13C]Ile rPrPC substrate is shown by Coomassie Blue staining of an SDS-polyacrylamide gel in lane 1 with molecular mass standards labeled in kDa to the left. Immunoblots of 263K BH-seeded [1-13C]Ile rPrP RT-QuIC product is shown before and after PK digestion in the presence or absence of Sarkosyl in lanes 2–6. For comparison, the PK-treated product of rPrP amyloid fibrils formed under unseeded destabilizing conditions as used in Ref. is shown in lane 7. C-terminal PrP antiserum R20 was used for immunoblotting. B, ultrastructure of negatively stained 263K BH-seeded RT-QuIC amyloid by transmission electron microscopy (bar, 20 nm).
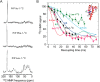
FIGURE 2.
13C ssNMR analysis of 263K BH-seeded RT-QuIC products. A, one-dimensional NMR spectra of 263K BH-seeded amyloid of specifically labeled rPrP. Negative shifts are indicative of β-sheet structure. B, dipolar recoupling experiments using PITHIRDS-CT ssNMR. Solid lines indicate standard decay rates for the given intermolecular distances of 0.4–0.7 nm. Lines with markers indicate observed decay rate for the given labeled amino acid within RT-QuIC products. Inset shows the location of labeled amino acids (by color) within PrPC (Protein Data Bank code 1AG2).
FIGURE 3.
Fibril linearity requires extended flattened structure. A, theoretical diagrammatic parallel in-register intermolecular β-sheet model for residues 90–231 PrPRes amyloid. B, destabilizing effects of combining a parallel in-register β-sheet architecture in the C-terminal ND hairpin (A and B, ND Hairpin) with two-tiered β-helices toward the N terminus (B, left side). The different thicknesses of these two architectures (β-helix versus parallel intermolecular β-sheet) within monomers would not appear to allow growth of stable linear fibrils, leaving major gaps along the fibril axis on the C-terminal side (B). The figure in A is adapted from Ref. .
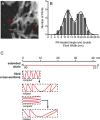
FIGURE 4.
Cross-sections of PrPRes amyloid fibrils. A, STEM imaging of PK-treated brain-derived GPI-anchorless fibrils (22L strain) (bar, 100 nm). Single and double red arrowheads indicate apparent single and double fibrils, respectively. B, fibril width distribution measured from STEM images and fit to a Sum of Two Gaussians linear regression to define two populations of apparent single and double fibrils. C, scale diagrams of the length of the PrP(90–231) polypeptide as an extended linear chain relative to potential folded architectures that would be required to fit such a polypeptide within the fibril widths given a coplanar parallel intermolecular β-sheet architecture. Yellow bars represent the native disulfide.
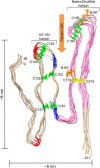
FIGURE 5.
Model of an octameric PrPRes amyloid segment subject to the experimental constraints outlined in text. The view is looking down the axis of an energy-minimized octamer of mouse PrP(90–231) molecules stacked parallel in-register and with the main hairpins (denoted on the model) defined by either the natural disulfide or the artificial disulfides (yellow or green, respectively) that have been shown to be compatible with PrPSc formation (46). The segment that is known to form parallel in-register intermolecular β-sheet in unseeded rPrP amyloid fibrils (see text) is pink. Also labeled are potential YYR antibody epitopes (blue) and PK cleavage sites (red) that, at least under some conditions, are preferentially exposed in PrPSc. Asn-linked glycosylation sites (orange) and the glycan cleft (orange arrow) are also indicated. Size constraints are shown on the outside of the model.
FIGURE 6.
Energy-minimized states of three distinct models of an octameric segment of PrPRes amyloid based on permutations of parallel in-register β-sheet architectures. In each view the individual monomers are designated by a different color with the broader arrows designating predicted β-strands. The end-on view is looking down the fibril axis. Arrows indicate the rotation of the fibril for the alternative views. A,
P
arallel
I
n-
R
egister
I
ntermolecular
β
-
S
heet
-A
(PIRIBS-A) model. B, PIRIBS-B model in which the 127–161 hairpin constrained by the artificial disulfides are inverted relative to the natural disulfide hairpin. This model also depicts the possibility that the extended C-terminal domain depicted in A might instead be folded back against the natural disulfide hairpin. C, PIRIBS-B model, which has an additional N-terminal loop at residue 127 compared with PIRIBS-B. In the latter model, the deletion (Δ141–176) that has been shown to be compatible with the formation of PrPSc “miniprions” (60) is shown in black. For all models, the N and C termini are denoted, as are the hairpins, the residues that define the hairpins, and the glycan cleft (orange arrows).
FIGURE 7.
Molecular dynamics simulations of the models shown in Fig. 6, retaining the mutant cysteines and disulfide bonds. A, PIRIBS-A model after 63 ns. B, PIRIBS-B model after 100 ns. C, PIRIBS-C model after 100 ns.
FIGURE 8.
Molecular dynamics simulations of the models shown in Fig. 6 with the mutant cysteines reverted to their wild-type residues. A, PIRIBS-A model after 63 ns. B, PIRIBS-B model after 100 ns. C, PIRIBS-C model after 155 ns.
FIGURE 9.
Location of isotope-labeled residues within the new PIRIBS-A model of PrPRes. An energy-minimized model as depicted in Fig. 5 but using the hamster PrP sequence for comparison with the ssNMR data, showing β-sheets (blue) and the positions of the Ile (magenta), Phe (black), and Leu (green) residues that were 13C-labeled for the ssNMR analyses before (A) and after (B) 163-ns molecular dynamics simulation.
FIGURE 10.
Celery stalk or half-pipe appearance of brain-derived GPI-anchorless (A, C (upper), and D) and wild-type PrPRes (B and C (lower)) amyloid (22L strain) by negative stain transmission electron microscopy. Flattened bends that have been observed in extended fibrils are marked with arrowheads. Red arrows designate the gap that was previously thought to be the spacing between individual protofilaments (14) but that we now think represents the expandable trough between the major hairpins (as diagrammed in C, expansion). Blue arrows flank the edges of what we perceive to be individual fibrils. A–C are adapted with permission from Ref. . All scale bars, 100 nm.
Similar articles
- Molecular structures of amyloid and prion fibrils: consensus versus controversy.
Tycko R, Wickner RB. Tycko R, et al. Acc Chem Res. 2013 Jul 16;46(7):1487-96. doi: 10.1021/ar300282r. Epub 2013 Jan 7. Acc Chem Res. 2013. PMID: 23294335 Free PMC article. Review. - The α-helical C-terminal domain of full-length recombinant PrP converts to an in-register parallel β-sheet structure in PrP fibrils: evidence from solid state nuclear magnetic resonance.
Tycko R, Savtchenko R, Ostapchenko VG, Makarava N, Baskakov IV. Tycko R, et al. Biochemistry. 2010 Nov 9;49(44):9488-97. doi: 10.1021/bi1013134. Biochemistry. 2010. PMID: 20925423 Free PMC article. - Mechanisms of Strain Diversity of Disease-Associated in-Register Parallel β-Sheet Amyloids and Implications About Prion Strains.
Taguchi Y, Otaki H, Nishida N. Taguchi Y, et al. Viruses. 2019 Jan 28;11(2):110. doi: 10.3390/v11020110. Viruses. 2019. PMID: 30696005 Free PMC article. Review. - Core structure of amyloid fibrils formed by residues 106-126 of the human prion protein.
Walsh P, Simonetti K, Sharpe S. Walsh P, et al. Structure. 2009 Mar 11;17(3):417-26. doi: 10.1016/j.str.2008.12.018. Structure. 2009. PMID: 19278656 - Conformational properties of prion strains can be transmitted to recombinant prion protein fibrils in real-time quaking-induced conversion.
Sano K, Atarashi R, Ishibashi D, Nakagaki T, Satoh K, Nishida N. Sano K, et al. J Virol. 2014 Oct;88(20):11791-801. doi: 10.1128/JVI.00585-14. Epub 2014 Jul 30. J Virol. 2014. PMID: 25078700 Free PMC article.
Cited by
- Multifaceted Role of Sialylation in Prion Diseases.
Baskakov IV, Katorcha E. Baskakov IV, et al. Front Neurosci. 2016 Aug 8;10:358. doi: 10.3389/fnins.2016.00358. eCollection 2016. Front Neurosci. 2016. PMID: 27551257 Free PMC article. Review. - A Structural and Functional Comparison Between Infectious and Non-Infectious Autocatalytic Recombinant PrP Conformers.
Noble GP, Wang DW, Walsh DJ, Barone JR, Miller MB, Nishina KA, Li S, Supattapone S. Noble GP, et al. PLoS Pathog. 2015 Jun 30;11(6):e1005017. doi: 10.1371/journal.ppat.1005017. eCollection 2015 Jun. PLoS Pathog. 2015. PMID: 26125623 Free PMC article. - Bank Vole Prion Protein As an Apparently Universal Substrate for RT-QuIC-Based Detection and Discrimination of Prion Strains.
Orrú CD, Groveman BR, Raymond LD, Hughson AG, Nonno R, Zou W, Ghetti B, Gambetti P, Caughey B. Orrú CD, et al. PLoS Pathog. 2015 Jun 18;11(6):e1004983. doi: 10.1371/journal.ppat.1004983. eCollection 2015 Jun. PLoS Pathog. 2015. PMID: 26086786 Free PMC article. - Synthetic β-sheets mimicking fibrillar and oligomeric structures for evaluation of spectral X-ray scattering technique for biomarker quantification.
Suresh K, Dahal E, Badano A. Suresh K, et al. Cell Biosci. 2024 Feb 19;14(1):26. doi: 10.1186/s13578-024-01208-6. Cell Biosci. 2024. PMID: 38374092 Free PMC article. - Full atomistic model of prion structure and conversion.
Spagnolli G, Rigoli M, Orioli S, Sevillano AM, Faccioli P, Wille H, Biasini E, Requena JR. Spagnolli G, et al. PLoS Pathog. 2019 Jul 11;15(7):e1007864. doi: 10.1371/journal.ppat.1007864. eCollection 2019 Jul. PLoS Pathog. 2019. PMID: 31295325 Free PMC article.
References
- Caughey B., Baron G. S. (2006) Prions and their partners in crime. Nature 443, 803–810 - PubMed
- Linden R., Martins V. R., Prado M. A., Cammarota M., Izquierdo I., Brentani R. R. (2008) Physiology of the prion protein. Physiol. Rev. 88, 673–728 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials