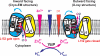Visualizing the kinetic power stroke that drives proton-coupled zinc(II) transport - PubMed (original) (raw)
Visualizing the kinetic power stroke that drives proton-coupled zinc(II) transport
Sayan Gupta et al. Nature. 2014.
Abstract
The proton gradient is a principal energy source for respiration-dependent active transport, but the structural mechanisms of proton-coupled transport processes are poorly understood. YiiP is a proton-coupled zinc transporter found in the cytoplasmic membrane of Escherichia coli. Its transport site receives protons from water molecules that gain access to its hydrophobic environment and transduces the energy of an inward proton gradient to drive Zn(II) efflux. This membrane protein is a well-characterized member of the family of cation diffusion facilitators that occurs at all phylogenetic levels. Here we show, using X-ray-mediated hydroxyl radical labelling of YiiP and mass spectrometry, that Zn(II) binding triggers a highly localized, all-or-nothing change of water accessibility to the transport site and an adjacent hydrophobic gate. Millisecond time-resolved dynamics reveal a concerted and reciprocal pattern of accessibility changes along a transmembrane helix, suggesting a rigid-body helical re-orientation linked to Zn(II) binding that triggers the closing of the hydrophobic gate. The gated water access to the transport site enables a stationary proton gradient to facilitate the conversion of zinc-binding energy to the kinetic power stroke of a vectorial zinc transport. The kinetic details provide energetic insights into a proton-coupled active-transport reaction.
Figures
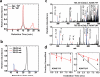
Fig. 1. Radiolytic labeling and mass spectrometric analysis
a: Size-exclusion HPLC chromatograms of apo-YiiP before irradiation and zinc-YiiP after irradiation. b: Examples for quantification of radiolytic labeling by LC-MS; extracted ion-count chromatograms of singly protonated, unmodified (749.33 m/z Da, black), carbonylated (+14 Da mass shift, 763.33 m/z, red) and hydroxylated (+ 16 Da mass sift, 765.33 m/z , blue) peptide ADMLHY. c: Examples for identification of modified residues by tandem mass spectrometry of the cabonylated and hydroxylated ADMLHY with peak assignments (red arrow and blue line) confirming L152 and M151 modification, respectively. d: Does-responses showing reciprocal solvent accessibility changes at L152 and M151 sites in apo-YiiP (red) and zinc-YiiP (black). Solid lines represent least-squares fits of the means of dose dependent data and the error bar represents standard error from 4-6 independent measurements.
Fig. 2. Quantification of water accessibility changes
a: Water accessibility changes in response to Zn(II) binding measured by the ratio of labeling rates for residues with an increase (blue), decrease (red) or no change (grey) in water accessibility after a rapid Zn(II) exposure. The labeling rate for each site as indicated is summarized in Extended Data Table 1, and the error bar represents standard error from 4-6 independent measurements. b: Residues with a partial water accessibility change in response to zinc binding. TM5 is colored in red. Z1 and Z2 (magenta spheres) represent bound zinc ions. Arrow indicates a putative zinc transport pathway from the cytoplasm through the L152 gate to the transport-site.
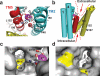
Fig. 3. L152 controls the opening of an inter-cavity water portal
a: A structural water molecule (W, red sphere) near the transport-site occupied by a tetrahedral coordinated Zn(II) (Z1, magenta sphere), viewed from the periplasm. Relevant residues are drawn in sticks and labeled accordingly. TM5 is colored in red as indicated. b: Intracellular and extracellular cavity as outlined by dash lines. c: L152 gate viewed from the extracellular cavity along the arrow as indicated in b. The sidechains of L152, I90 and coordination residues in the transport-site (sticks) are excluded from the protein surface drawing. M197 is shown as a yellow patch at the cytoplasmic entrance to the inter-cavity portal. d: L152 gate viewed from the intracellular cavity along the arrow as indicated in b. M151 and M197 are visible as yellow patches on the protein surface.
Fig.4. Kinetics of water accessibility changes
a: An example of extracted ion chromatograms from the unmodified and modified M197 in peptide 197-208. The data were smoothed by a low-passing filter and normalized to the peak height of respective unmodified species. The arrow indicates a progressive decrease of the modified peaks as a function of the reaction time. b: Time courses of water accessibility change for indicated residues. The solid line represents a single exponential fit of the time course of the unmodified fraction with a fitted rate constant (k) presented as mean ± standard error from six independent measurements.
Fig. 5. Schematic representation of zinc-for-proton exchange
based on two existing structural models with the L152 gate open or closed as indicated. The protein conformational change alternates the membrane facing, on-off mode of zinc coordination and protonation-deprotonation of the transport-site in a coordinated fashion.
Extended Data Figure 1. Steady state and time resolved synchrotron X-ray radiolysis
Experimental scheme for obtaining steady state dose plots. b: Experimental schemes for obtaining the time course of water accessibility change.
Extended Data Figure 2. a. Mass spectrometry sequence coverage
The detectable peptides, undetectable residues and coordination residues of the transport-site are shown in orange, green and red respectively. Transmembrane helixes (TMs) are underlined as indicated. b: Mapping detectable proteolytic peptides to the YiiP crystal structure. Detectable and undetectable peptides in one protomer of a YiiP homodimer are colored in orange and green, respectively. The sidechains of detectable residues located between two cavities are shown in surface representation and colored in red. Bound zinc ions are shown as magenta spheres.
Extended Data Figure 3. Does-responses for modified sites in apo-YiiP (red) and zinc-YiiP (black)
Solid lines represent least-squares fits of the dose dependent data as described in Methods. The reactivity rate for each site as indicated is summarized in Extended Data Table 1. Note, the linear does-response on a logarithm scale indicates negligible radiation damage by 10 ms irradiation to all sites except M151, M197 and P286C287 which reached saturation at 5 ms. In these cases, the 10 ms data points were not used in linear regression. Dose response plots for M151 and L152 is shown in Fig 1d. Zinc binding to a solvent exposed zinc site located on the cytoplasmic membrane surface (Z2, Fig. 2b) yielded 38-67% reductions in oxidative modification of neighboring L64, P66, D68, D69, H71 and F73 within the peptide SLQPADDNHSF (Extended Data Table 1). A marginal 20% reduction in water accessibility change by zinc binding was also localized to W172 and W175 within an extracellular loop connecting TM5 and TM6 (Fig. 2b). This loop is disordered in the crystal structure. Structural flexibility likely allows for distinct loop conformations in response to zinc binding. A total of six modified sites were identified in CTD (Extended Data Table 1). Two sites (M251, P286/C287) with reduced water accessibility for zinc-YiiP are located near the binuclear zinc binding site at the CDT-CDT interface. Zinc binding may partially protect these sites from labeling, but the protection was incomplete because of the solvent exposure. Two sites on the CTD protein surface (W225, P257/L258) showed no detectable change as expected for fully exposed residues. Still two more sites exhibited an increase in water accessibility for zinc-YiiP. The first site (D207-L210) was mapped to a TMD-CDT linker which is involved in a hinge-like conformational change in response to zinc binding. The second site involved M262 on the CDT surface. There was no obvious explanation for the increase of water accessibility to this methionine residue.
Extended Data Figure 4. Sequence conservation of the inter-cavity seal
Residues involved in TM5→TM3-TM6 packing are marked by black asterisks in a CDF sequence alignment. Conserved and homologous residues are colored in magenta and light-blown. Magenta, cyan and black dots indicate residues involved in zinc coordination, dimerization contacts and the interlocked (Lys77-Asp207)2 salt-bridges, respectively. Dashed lines in two human ZnT sequences represent omitted residues in a loop (IL2) between TM4 and TM5. Red arrow indicates the position of the R325W mutation in human ZnT8.
Extended Data Figure 5. Expression and size-exclusion analysis of purified L152 mutants
L152 was substituted by an A, D, F, G, I, M or R residue to evaluate the effect of L152 mutations on structural stability. a: Western-blot analysis of the expression of YiiP and L152 mutants as indicated. Only a L152R point mutation caused a modest reduction of protein expression whereas other L152 substitutions and wild type YiiP showed a similar level of expression based on western-blot detection of His-tagged proteins in membrane vesicles using a monoclonal antibody against the poly-histidine tag. b: Eluted protein peaks for YiiP and L152 mutants as indicated after the removal of protein aggregates by ultracentrifugation. YiiP and L152 mutants were solubilized by DDM and purified by Ni-NTA affinity chromatography and size exclusion HPLC. The purified wild type YiiP remained stable in DDM micelles for weeks. Sizing HPLC analysis showed a monodisperse YiiP peak followed by a minor detergent peak eluted at expected retention times. In sharp contrast, purified L152 mutants rapidly denatured, forming lager protein aggregates. After removing the aggregates by ultracentrifugation, none of the purified L152A, D, F, G, and R became detectable by sizing HPLC. Two conserved L152 substitutions, L152I and L152M showed a significantly reduced peak volume within 48 hours of DDM solubilization. A prolonged DDM solubilization led to complete denaturation of L152I and L152M while the wild type YiiP remained stable under the same experimental condition. Thus, L152 is critically important to the protein stability in detergent micelles. The lack of protein stability in detergent solution precluded functional reconstitution and characterization of purified L152 mutants.
Similar articles
- The mechanism of mammalian proton-coupled peptide transporters.
Lichtinger SM, Parker JL, Newstead S, Biggin PC. Lichtinger SM, et al. Elife. 2024 Jul 23;13:RP96507. doi: 10.7554/eLife.96507. Elife. 2024. PMID: 39042711 Free PMC article. - Thermodynamic studies of the mechanism of metal binding to the Escherichia coli zinc transporter YiiP.
Chao Y, Fu D. Chao Y, et al. J Biol Chem. 2004 Apr 23;279(17):17173-80. doi: 10.1074/jbc.M400208200. Epub 2004 Feb 11. J Biol Chem. 2004. PMID: 14960568 - Structure of the zinc transporter YiiP.
Lu M, Fu D. Lu M, et al. Science. 2007 Sep 21;317(5845):1746-8. doi: 10.1126/science.1143748. Epub 2007 Aug 23. Science. 2007. PMID: 17717154 - From membrane to molecule to the third amino acid from the left with a membrane transport protein.
Kaback HR, Wu J. Kaback HR, et al. Q Rev Biophys. 1997 Nov;30(4):333-64. doi: 10.1017/s0033583597003387. Q Rev Biophys. 1997. PMID: 9634651 Review. - The lactose permease of Escherichia coli: overall structure, the sugar-binding site and the alternating access model for transport.
Abramson J, Smirnova I, Kasho V, Verner G, Iwata S, Kaback HR. Abramson J, et al. FEBS Lett. 2003 Nov 27;555(1):96-101. doi: 10.1016/s0014-5793(03)01087-1. FEBS Lett. 2003. PMID: 14630326 Review.
Cited by
- PfeT, a P1B4 -type ATPase, effluxes ferrous iron and protects Bacillus subtilis against iron intoxication.
Guan G, Pinochet-Barros A, Gaballa A, Patel SJ, Argüello JM, Helmann JD. Guan G, et al. Mol Microbiol. 2015 Nov;98(4):787-803. doi: 10.1111/mmi.13158. Epub 2015 Sep 10. Mol Microbiol. 2015. PMID: 26261021 Free PMC article. - Structural Elements in the Transmembrane and Cytoplasmic Domains of the Metal Transporter SLC30A10 Are Required for Its Manganese Efflux Activity.
Zogzas CE, Aschner M, Mukhopadhyay S. Zogzas CE, et al. J Biol Chem. 2016 Jul 29;291(31):15940-57. doi: 10.1074/jbc.M116.726935. Epub 2016 Jun 15. J Biol Chem. 2016. PMID: 27307044 Free PMC article. - An automated liquid jet for fluorescence dosimetry and microsecond radiolytic labeling of proteins.
Rosi M, Russell B, Kristensen LG, Farquhar ER, Jain R, Abel D, Sullivan M, Costello SM, Dominguez-Martin MA, Chen Y, Marqusee S, Petzold CJ, Kerfeld CA, DePonte DP, Farahmand F, Gupta S, Ralston CY. Rosi M, et al. Commun Biol. 2022 Aug 25;5(1):866. doi: 10.1038/s42003-022-03775-1. Commun Biol. 2022. PMID: 36008591 Free PMC article. - Structural insights into the calcium-coupled zinc export of human ZnT1.
Sun C, He B, Gao Y, Wang X, Liu X, Sun L. Sun C, et al. Sci Adv. 2024 Apr 26;10(17):eadk5128. doi: 10.1126/sciadv.adk5128. Epub 2024 Apr 26. Sci Adv. 2024. PMID: 38669333 Free PMC article. - Protein Footprinting: Auxiliary Engine to Power the Structural Biology Revolution.
Chance MR, Farquhar ER, Yang S, Lodowski DT, Kiselar J. Chance MR, et al. J Mol Biol. 2020 Apr 17;432(9):2973-2984. doi: 10.1016/j.jmb.2020.02.011. Epub 2020 Feb 21. J Mol Biol. 2020. PMID: 32088185 Free PMC article. Review.
References
- Grass G, et al. FieF (YiiP) from Escherichia coli mediates decreased cellular accumulation of iron and relieves iron stress. Arch Microbiol. 2005;183:9–18. - PubMed
- Chao Y, Fu D. Thermodynamic studies of the mechanism of metal binding to the Escherichia coli zinc transporter YiiP. J Biol Chem. 2004;279:17173–17180. - PubMed
- Wei Y, Fu D. Selective metal binding to a membrane-embedded aspartate in the Escherichia coli metal transporter YiiP (FieF). J Biol Chem. 2005;280:33716–33724. - PubMed
- Wei Y, Fu D. Binding and transport of metal ions at the dimer interface of the Escherichia coli metal transporter YiiP. J Biol Chem. 2006;281:23492–23502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-EB-09688/EB/NIBIB NIH HHS/United States
- R01GM065137/GM/NIGMS NIH HHS/United States
- P30 DK089502/DK/NIDDK NIH HHS/United States
- P30-EB-09998/EB/NIBIB NIH HHS/United States
- R01 EB009688/EB/NIBIB NIH HHS/United States
- P30 EB009998/EB/NIBIB NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- R01 GM065137/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases