Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells - PubMed (original) (raw)
Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells
Chuan Wu et al. Immunity. 2014.
Abstract
The β-galactoside-binding protein galectin-9 is critical in regulating the immune response, but the mechanism by which it functions remains unclear. We have demonstrated that galectin-9 is highly expressed by induced regulatory T cells (iTreg) and was crucial for the generation and function of iTreg cells, but not natural regulatory T (nTreg) cells. Galectin-9 expression within iTreg cells was driven by the transcription factor Smad3, forming a feed-forward loop, which further promoted Foxp3 expression. Galectin-9 increased iTreg cell stability and function by directly binding to its receptor CD44, which formed a complex with transforming growth factor-β (TGF-β) receptor I (TGF-βRI), and activated Smad3. Galectin-9 signaling was further found to regulate iTreg cell induction by dominantly acting through the CNS1 region of the Foxp3 locus. Our data suggest that exogenous galectin-9, in addition to being an effector molecule for Treg cells, acts synergistically with TGF-β to enforce iTreg cell differentiation and maintenance.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
Figure 1. Galectin-9 promotes Foxp3 expression during iTreg cell differentiation
(A) The percentage of Foxp3+ Treg cells in mesenteric LN (mLN) and lamina propria (LP) was determined by flow cytometry; (B) Flow cytometry analysis of CD4+ T cells from the LP of WT or Lgals9−/− mice; (C) Activated naïve CD4+ T cells were stimulated with TGF-β and/or recombinant galectin-9. The frequency of Foxp3+ cells was determined by flow cytometry; CD45.1+ WT and CD45.2+ Lgals9−/− naïve T cells were cultured (D) separately or (E) together in the presence of TGF-β. The frequency of Foxp3+ cells was then determined by flow cytometry; OT-II Rag2−/− and OT-II Rag2−/−Lgals9−/− CD45.2+CD4+ T cells were transferred into congenic CD45.1 (F) Lgals9−/− or (G) WT recipient mice, followed by administration of OVA in the drinking water for 5 days. CD45.2+Foxp3+ iTreg cells from Peyer’s patches (PP) and LP of Lgals9−/− recipient mice were determined by flow cytometry. Data are representative of three independent experiments with n≥4 mice each group.
Figure 2. Smad3 regulates galectin-9 during iTreg cell differentiation
(A) Naïve CD4+CD44loCD62L+CD25− T cells from WT mice were differentiated into different T cell subsets. Quantitative real-time PCR analysis of Lgals9 mRNA in different subsets is presented relative to the expression of GAPDH mRNA; (B) Protein expression of galectin-9 was determined in different T cell subsets by flow cytometry; (C) The binding of Smad3 to the Lgals9 promoter in Th0 and iTreg cells was assayed by ChIP-PCR. Six horizontal bars represent the locations of Smad3 binding sites on Lgals9 locus detected by real-time PCR; (D–E) Luciferase assay using a Lgals9 promoter-driven reporter in EL4 LAF cells transfected with a control or Smad3-expressing vector under the indicated conditions. Promoter activity was determined 24 h after the addition of stimuli; (F) mRNA and (G) protein expression levels of Foxp3 and galectin-9 within WT and Smad3−/− iTreg cells; Data are representative of three independent experiments with n ≥ 4 mice each group (A–B, F–G) or are pooled from three independent experiments (C–E). *P< 0.05 and **P< 0.01 (Student’s _t_-test, error bars, SD).
Figure 3. Lgals9−/− iTreg cells have reduced suppressive activity
(A) Proliferation of WT naïve CD4+ T cells in the presence of anti-CD3 and anti-CD28 and GFP+ (Foxp3+) iTreg cells from Foxp3GFP and Foxp3GFPLgals9−/− mice. Data shown are presented as mean [3H]-thymidine incorporation; (B) The expression of indicated co-stimulatory molecules on Foxp3GFP and Foxp3GFPLgals9−/− CD4+GFP+ iTreg cells was determined by flow cytometry; (C) Body weight of Rag2−/−Lgals9−/− mice transferred with WT CD4+CD25−CD45RBhi T cells with or without WT or Lgals9−/− Foxp3+ (GFP) iTreg cells; (D) Hematoxylin and eosin staining of colon samples from the different groups as in (C) (original magnification, ×20); (E) Flow cytometry of IL-17 and IFN-γ secretion by CD4+ T cells isolated in spleen and LP from indicated groups as in (C) 10 weeks after colitis induction. The data are representative of three independent experiments with n ≥ 4 mice each group.* P< 0.05 (Student’s _t_-test, error bars, SD).
Figure 4. Galectin-9 enhances iTreg cell stability
(A) Purified GFP+ iTreg from Foxp3GFP and Foxp3GFPLgals9−/− mice were i.v. transferred into Rag2−/−Lgals9−/− recipient mice. 2 and 5 days after transfer, frequency of GFP+ cells were determined by flow cytometry; (B) IL-17 and IFN-γ production in the GFP− fraction in (A) were determined by flow cytometry; (C) Body weight of Rag2−/−Lgals9−/− mice transferred i.p. with CD4+CD25−CD45RBhiRFP−YFP− T cells from WT or Lgals9−/− FM mice; (D) Flow cytometry of IL-17 and IFN-γ, YFP and RFP by CD4+ T cells isolated in LP from indicated groups as in (C) 10 weeks after colitis induction. The data are representative of three independent experiments with n ≥ 5 mice each group. *P < 0.05, **P< 0.01 (Student’s _t_-test, error bars, SD).
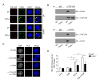
Figure 5. CD44 mediates galectin-9 signaling during iTreg differentiation
(A) Confocal images of WT or Lgals9−/− Th0 and iTreg cells stained with anti-TGF-βRI and anti-CD44 antibodies; (B) Immunoprecipitation (with control IgG, anti-TGF-βRI or anti-CD44) of lysates of WT and Cd44−/− iTreg cells, followed by immunoblot analysis with the indicated antibodies; (C) TGF-βRI and CD44 association was visualized in iTreg cells with an in situ proximity ligation assay. Punctate staining (red) indicates a TGF-βRI-CD44 interaction as detected by the assay; (D) Anti-CD3 and anti-CD28 activated WT and Cd44−/− naïve CD4+ T cells were stimulated with TGF-β or recombinant galectin-9 and TGF-βRI kinase activity was determined. Data are representative of two independent experiments (A–C) or are pooled from three independent experiments (D) with n≥4 mice each group.* P< 0.05 (Student’s _t_-test, error bars, SD).
Figure 6. CNS1 region is essential for galectin-9 signaling within iTreg but not nTreg cells
(A) The binding of Smad3 and AcH4 to the Foxp3 CNS1 region from sorted GFP+T cells of Figure S6C from Foxp3GFP and Foxp3GFPLgals9−/− iTreg cells was determined by ChIP-PCR; (B) The binding of AcH4 to the Foxp3 CNS1 region from activated naïve T cells as in Figure S6D was determined by ChIP-PCR; (C) EL4 LAF cells were transfected with a Foxp3 promoter construct with or without the CNS1 region and stimulated with anti-CD3 and anti-CD28 antibodies in the presence of the indicated stimulations. Luciferase activity was measured 48 hours later. Data are representative of two independent experiments; (D) Anti-CD3 and anti-CD28 activated naïve CD4+ T cells from WT or Foxp3_Δ_CNS1 mice were stimulated with different combinations of TGF-β and recombinant galectin-9 and the frequency of Foxp3+ cells was determined by flow cytometry; (E) Chimeric mice were generated by transferring WT or Foxp3_Δ_CNS1 BM into WT or Lgals9−/− host mice. 10 weeks after reconstitution, the frequency of Foxp3+Nrp1lo iTreg cells or Foxp3+Nrp1hi nTreg cells in PP and LP was determined by flow cytometry. Data are pooled from three independent experiments (A–C) or are representative of two independent experiments (D–E) with n≥4 mice each group. *P < 0.05 and **P < 0.01 (Student’s _t_-test, error bars, SD).
Figure 7. Galectin-9-CD44 interaction regulates iTreg differentiation and function
(A) Activated WT naïve CD4+ T cells were stimulated with combinations of anti-CD44 antibody and recombinant galectin-9 with or without TGF-β for 3 days. The frequency of Foxp3+ cells was determined by flow cytometry; (B) Anti-CD3 and anit-CD28 activated naïve CD4+ T cells from WT or Cd44−/− mice were stimulated with different combinations of TGF-β and recombinant galectin-9 and the frequency of Foxp3+ cells was determined by flow cytometry; (C) Heat map displaying nanostring data, fold change of selected gene subsets in the experimental settings: Cd44−/− versus Lgals9−/− versus WT iTreg cells; (D) Proliferation of naïve CD4+ T cells in the presence of anti-CD3 and anti-CD28 and GFP+ (Foxp3+) iTreg cells from WT or Cd44−/− mice. Data shown are presented as mean [3H]-thymidine incorporation (cpm ± SEM, performed in triplicate); (E) Body weight of Rag2−/− mice transferred with WT CD4+CD25−CD45RBhi T cells with or without WT or Cd44−/− Foxp3+ (GFP) iTreg cells. Data are representative of three independent experiments with n ≥ 5 mice each group. *P < 0.05 (Student’s _t_-test, error bars, SD).
Comment in
- T cells are Smad'ly in love with galectin-9.
Cummings RD. Cummings RD. Immunity. 2014 Aug 21;41(2):171-3. doi: 10.1016/j.immuni.2014.08.001. Immunity. 2014. PMID: 25148018
Similar articles
- T cells are Smad'ly in love with galectin-9.
Cummings RD. Cummings RD. Immunity. 2014 Aug 21;41(2):171-3. doi: 10.1016/j.immuni.2014.08.001. Immunity. 2014. PMID: 25148018 - Dichotomous role of TGF-β controls inducible regulatory T-cell fate in allergic airway disease through Smad3 and TGF-β-activated kinase 1.
Joetham A, Schedel M, Ning F, Wang M, Takeda K, Gelfand EW. Joetham A, et al. J Allergy Clin Immunol. 2020 Mar;145(3):933-946.e4. doi: 10.1016/j.jaci.2019.09.032. Epub 2019 Oct 15. J Allergy Clin Immunol. 2020. PMID: 31626843 Free PMC article. - Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway.
Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, Filbey KJ, Finney CA, Greenwood EJ, Knox DP, Wilson MS, Belkaid Y, Rudensky AY, Maizels RM. Grainger JR, et al. J Exp Med. 2010 Oct 25;207(11):2331-41. doi: 10.1084/jem.20101074. Epub 2010 Sep 27. J Exp Med. 2010. PMID: 20876311 Free PMC article. - Revisiting regulatory T cells in type 1 diabetes.
Zhang Y, Bandala-Sanchez E, Harrison LC. Zhang Y, et al. Curr Opin Endocrinol Diabetes Obes. 2012 Aug;19(4):271-8. doi: 10.1097/MED.0b013e328355a2d5. Curr Opin Endocrinol Diabetes Obes. 2012. PMID: 22732485 Review. - Transforming growth factor-beta: an important role in CD4+CD25+ regulatory T cells and immune tolerance.
Zhang L, Yi H, Xia XP, Zhao Y. Zhang L, et al. Autoimmunity. 2006 Jun;39(4):269-76. doi: 10.1080/08916930600753903. Autoimmunity. 2006. PMID: 16891215 Review.
Cited by
- Galectin-1 fosters an immunosuppressive microenvironment in colorectal cancer by reprogramming CD8+ regulatory T cells.
Cagnoni AJ, Giribaldi ML, Blidner AG, Cutine AM, Gatto SG, Morales RM, Salatino M, Abba MC, Croci DO, Mariño KV, Rabinovich GA. Cagnoni AJ, et al. Proc Natl Acad Sci U S A. 2021 May 25;118(21):e2102950118. doi: 10.1073/pnas.2102950118. Proc Natl Acad Sci U S A. 2021. PMID: 34006646 Free PMC article. - Enriched circulating and tumor-resident TGF-β+ regulatory B cells in patients with melanoma promote FOXP3+ Tregs.
Harris RJ, Willsmore Z, Laddach R, Crescioli S, Chauhan J, Cheung A, Black A, Geh JLC, MacKenzie Ross AD, Healy C, Tsoka S, Spicer J, Lacy KE, Karagiannis SN. Harris RJ, et al. Oncoimmunology. 2022 Jul 28;11(1):2104426. doi: 10.1080/2162402X.2022.2104426. eCollection 2022. Oncoimmunology. 2022. PMID: 35909944 Free PMC article. - The Role of Nucleic Acid Sensing in Controlling Microbial and Autoimmune Disorders.
Matz KM, Guzman RM, Goodman AG. Matz KM, et al. Int Rev Cell Mol Biol. 2019;345:35-136. doi: 10.1016/bs.ircmb.2018.08.002. Epub 2018 Sep 25. Int Rev Cell Mol Biol. 2019. PMID: 30904196 Free PMC article. Review. - Precise and Rapid Validation of Candidate Gene by Allele Specific Knockout With CRISPR/Cas9 in Wild Mice.
Chao T, Liu Z, Zhang Y, Zhang L, Huang R, He L, Gu Y, Chen Z, Zheng Q, Shi L, Zheng W, Qi X, Kong E, Zhang Z, Lawrence T, Liang Y, Lu L. Chao T, et al. Front Genet. 2019 Feb 19;10:124. doi: 10.3389/fgene.2019.00124. eCollection 2019. Front Genet. 2019. PMID: 30838037 Free PMC article. - Novel T cell exhaustion gene signature to predict prognosis and immunotherapy response in thyroid carcinoma from integrated RNA-sequencing analysis.
Li Y, Wang Z, Lu F, Miao Y, Feng Q, Zhu W, Kang Q, Chen Y, Zhang Q. Li Y, et al. Sci Rep. 2024 Apr 10;14(1):8375. doi: 10.1038/s41598-024-58419-7. Sci Rep. 2024. PMID: 38600248 Free PMC article.
References
- Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW, Hirashima M, et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318:1141–1143. - PubMed
- Arikawa T, Saita N, Oomizu S, Ueno M, Matsukawa A, Katoh S, Kojima K, Nagahara K, Miyake M, Yamauchi A, et al. Galectin-9 expands immunosuppressive macrophages to ameliorate T-cell-mediated lung inflammation. European journal of immunology. 2010;40:548–558. - PubMed
- Arikawa T, Watanabe K, Seki M, Matsukawa A, Oomizu S, Sakata KM, Sakata A, Ueno M, Saita N, Niki T, et al. Galectin-9 ameliorates immune complex-induced arthritis by regulating Fc gamma R expression on macrophages. Clinical immunology. 2009;133:382–392. - PubMed
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01NS045937/NS/NINDS NIH HHS/United States
- P01AI056299/AI/NIAID NIH HHS/United States
- P01 AI056299/AI/NIAID NIH HHS/United States
- R01 NS045937/NS/NINDS NIH HHS/United States
- K01 DK090105/DK/NIDDK NIH HHS/United States
- K01DK090105/DK/NIDDK NIH HHS/United States
- P01 AI039671/AI/NIAID NIH HHS/United States
- P01 AI073748/AI/NIAID NIH HHS/United States
- P01AI039671/AI/NIAID NIH HHS/United States
- R01 NS030843/NS/NINDS NIH HHS/United States
- P01AI073748/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous