Development of novel three-dimensional printed scaffolds for osteochondral regeneration - PubMed (original) (raw)
Development of novel three-dimensional printed scaffolds for osteochondral regeneration
Benjamin Holmes et al. Tissue Eng Part A. 2015 Jan.
Abstract
As modern medicine advances, various methodologies are being explored and developed in order to treat severe osteochondral defects in joints. However, it is still very challenging to cure the osteochondral defects due to their poor inherent regenerative capacity, complex stratified architecture, and disparate biomechanical properties. The objective of this study is to create novel three-dimensional (3D) printed osteochondral scaffolds with both excellent interfacial mechanical properties and biocompatibility for facilitating human bone marrow mesenchymal stem cell (MSC) growth and chondrogenic differentiation. For this purpose, we designed and 3D printed a series of innovative bi-phasic 3D models that mimic the osteochondral region of articulate joints. Our mechanical testing results showed that our bi-phasic scaffolds with key structures have enhanced mechanical characteristics in compression (a maximum Young's modulus of 31 MPa) and shear (a maximum fracture strength of 5768 N/mm(2)) when compared with homogenous designs. These results are also correlated with numerical simulation. In order to improve their biocompatibility, the scaffolds' surfaces were further modified with acetylated collagen (one of the main components in osteochondral extracellular matrix). MSC proliferation results demonstrated that incorporation of a collagen, along with biomimetically designed micro-features, can greatly enhance MSC growth after 5 days in vitro. Two weeks' chondrogenic differentiation results showed that our novel scaffolds (dubbed "key" scaffolds), both with and without surface collagen modification, displayed enhanced chondrogenesis (e.g., 130%, 114%, and 236% increases in glycosaminoglycan, type II collagen deposition, and total protein content on collagen-modified key scaffolds when compared with homogeneous controls).
Figures
**FIG. 1.
A picture of our three-dimensional (3D) printer setup. Color images available online at
**FIG. 2.
Computer-aided design (CAD) images of our (1) large and (2) small pore model and (A) homogeneous, (B) bi-phasic, and (C) bi-phasic key featured designs. For bi- and key scaffolds, the bone layer is on the bottom (based on the orientation of this figure) and the cartilage layer is on the top. Color images available online at
**FIG. 3.
Schematic illustration of acetylated collagen linked on 3D printed bi-phasic key scaffold. Color images available online at
**FIG. 4.
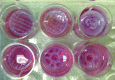
Images of 3D printed scaffolds with different internal geometry and pore density in mesenchymal stem cell (MSC) growth media. Small (top) and large (bottom) pore models, and (from left to right) homogeneous, bi-phasic, and bi-phasic key feature scaffolds. Color images available online at
**FIG. 5.
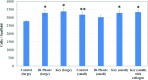
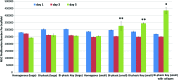
Compressive Young's modulus data for 3D printed scaffolds. Data are±standard error of the mean, _n_=5; *p<0.05 when compared with all homogenous and bi-phasic scaffolds; **p<0.05 when compared with all other scaffolds with small features; and #p<0.05 when compared with all other scaffolds. Color images available online at
**FIG. 6.
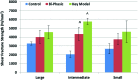
Shear fracture strength of 3D printed scaffolds, performed under wedge fracture shear testing. Data are±standard error of the mean, n_=5; ^_p<0.01 when compared with controls with intermediate pores. Color images available online at
**FIG. 7.
Scanning electron microscopy images of (A, B) unmodified and (C, D) acetylated collagen-modified 3D printed osteochondral scaffolds. (E) Contact angle analysis of pure poly lactic acid (PLA) and PLA with acetylated collagen type I, showing decreased contact angle on the collagen-modified PLA. Data are±standard error of the mean, _n_=10; *p<0.05 when compared with control. Color images available online at
**FIG. 8.
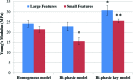
MSC adhesion on different 3D printed scaffolds. Data are±standard error of the mean, _n_=9; *p<0.05 when compared with control with large features and bi-phasic scaffold with small features. **p<0.05 when compared with control with large features. Color images available online at
**FIG. 9.
MSC proliferation in a variety of 3D printed PLA scaffolds with different internal structure and surface modification. Data are±standard error of the mean, _n_=9; *p<0.05 when compared with all other scaffolds and **p<0.05 when compared with all scaffolds with large features and homogenous controls with small features at day 5. Color images available online at
**FIG. 10.
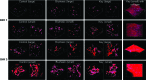
Confocal microscopy images of MSC growing on 3D printed scaffolds after 1 and 3 days of culture. Improved cell growth and spreading morphology are observed on smaller featured scaffolds. Color images available online at
**FIG. 11.
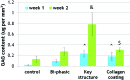
Glycosaminoglycan (GAG) synthesis in various 3D printed osteochondral scaffolds. Data are±standard error of the mean, n_=9; &p<0.05 when compared with all other scaffolds and $p<0.05 when compared with controls after 2 weeks; and ^_p<0.05 when compared with controls and bi-phasic scaffolds after 1 week. Color images available online at
**FIG. 12.
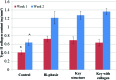
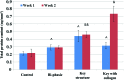
Collagen type II synthesis on 3D printed scaffolds. All groups showed improvement after 2 weeks of chondrogenic differentiation. Data are±standard error of the mean, _n_=9; *p<0.05 when compared with all other scaffolds at week 1 and ^ p<0.05 when compared with all other scaffolds at week 2. Color images available online at
**FIG. 13.
Total protein synthesis. Data are±standard error of the mean, n_=9; ^_p<0.05 when compared with controls, &p<0.05 when compared with all other scaffolds and &&p<0.05 when compared with bi-phasic and controls after 2 weeks. Color images available online at
**FIG. 14.
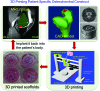
A flow chart illustrating 3D printing patient-specific scaffolds with designed internal structures for osteochondral defect treatment. Color images available online at
**FIG. 15.
A full knee cartilage layer CAD model and the respective 3D printed full knee construct. Color images available online at
Similar articles
- 3D-printed biphasic scaffolds for the simultaneous regeneration of osteochondral tissues.
Natarajan ABM, Sivadas VPD, Nair PDPD. Natarajan ABM, et al. Biomed Mater. 2021 Jul 29;16(5). doi: 10.1088/1748-605X/ac14cb. Biomed Mater. 2021. PMID: 34265754 - Cryogenic 3D printing of heterogeneous scaffolds with gradient mechanical strengths and spatial delivery of osteogenic peptide/TGF-β1 for osteochondral tissue regeneration.
Wang C, Yue H, Huang W, Lin X, Xie X, He Z, He X, Liu S, Bai L, Lu B, Wei Y, Wang M. Wang C, et al. Biofabrication. 2020 Mar 23;12(2):025030. doi: 10.1088/1758-5090/ab7ab5. Biofabrication. 2020. PMID: 32106097 - Precision 3D printed meniscus scaffolds to facilitate hMSCs proliferation and chondrogenic differentiation for tissue regeneration.
Deng X, Chen X, Geng F, Tang X, Li Z, Zhang J, Wang Y, Wang F, Zheng N, Wang P, Yu X, Hou S, Zhang W. Deng X, et al. J Nanobiotechnology. 2021 Dec 2;19(1):400. doi: 10.1186/s12951-021-01141-7. J Nanobiotechnology. 2021. PMID: 34856996 Free PMC article. - Osteochondral Regeneration With Anatomical Scaffold 3D-Printing-Design Considerations for Interface Integration.
Nedrelow DS, Townsend JM, Detamore MS. Nedrelow DS, et al. J Biomed Mater Res A. 2025 Jan;113(1):e37804. doi: 10.1002/jbm.a.37804. Epub 2024 Oct 10. J Biomed Mater Res A. 2025. PMID: 39387548 Review. - Therapeutic "Tool" in Reconstruction and Regeneration of Tissue Engineering for Osteochondral Repair.
Hu X, Xu J, Li W, Li L, Parungao R, Wang Y, Zheng S, Nie Y, Liu T, Song K. Hu X, et al. Appl Biochem Biotechnol. 2020 Jun;191(2):785-809. doi: 10.1007/s12010-019-03214-8. Epub 2019 Dec 21. Appl Biochem Biotechnol. 2020. PMID: 31863349 Review.
Cited by
- Cryogenic 3D printing of dual-delivery scaffolds for improved bone regeneration with enhanced vascularization.
Wang C, Lai J, Li K, Zhu S, Lu B, Liu J, Tang Y, Wei Y. Wang C, et al. Bioact Mater. 2020 Aug 12;6(1):137-145. doi: 10.1016/j.bioactmat.2020.07.007. eCollection 2021 Jan. Bioact Mater. 2020. PMID: 32817920 Free PMC article. - 3D Bioprinting for Organ Regeneration.
Cui H, Nowicki M, Fisher JP, Zhang LG. Cui H, et al. Adv Healthc Mater. 2017 Jan;6(1):10.1002/adhm.201601118. doi: 10.1002/adhm.201601118. Epub 2016 Dec 20. Adv Healthc Mater. 2017. PMID: 27995751 Free PMC article. Review. - A 3D printed nano bone matrix for characterization of breast cancer cell and osteoblast interactions.
Zhu W, Castro NJ, Cui H, Zhou X, Boualam B, McGrane R, Glazer RI, Zhang LG. Zhu W, et al. Nanotechnology. 2016 Aug 5;27(31):315103. doi: 10.1088/0957-4484/27/31/315103. Epub 2016 Jun 27. Nanotechnology. 2016. PMID: 27346678 Free PMC article. - Three-dimensional printing of nanomaterial scaffolds for complex tissue regeneration.
O'Brien CM, Holmes B, Faucett S, Zhang LG. O'Brien CM, et al. Tissue Eng Part B Rev. 2015 Feb;21(1):103-14. doi: 10.1089/ten.TEB.2014.0168. Epub 2014 Sep 16. Tissue Eng Part B Rev. 2015. PMID: 25084122 Free PMC article. Review. - 3D Bone Biomimetic Scaffolds for Basic and Translational Studies with Mesenchymal Stem Cells.
Sobacchi C, Erreni M, Strina D, Palagano E, Villa A, Menale C. Sobacchi C, et al. Int J Mol Sci. 2018 Oct 13;19(10):3150. doi: 10.3390/ijms19103150. Int J Mol Sci. 2018. PMID: 30322134 Free PMC article. Review.
References
- Castro N.J., Hacking S.A., and Zhang L.G.Recent progress in interfacial tissue engineering approaches for osteochondral defects. Ann Biomed Eng 40,1628, 2012 - PubMed
- Centers for Disease Control and Prevention. A National Public Health Agenda for Osteoarthritis 2010, Available at: www.cdc.gov/arthritis/docs/oaagenda.pdf
- Zhang L., and Webster T.J.Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nano Today 4,66, 2009
- Levine B., and Kanat I.O.Subchondral bone cysts, osteochondritis dissecans, and Legg-Calve-Perthes disease: a correlation and proposal of their possible common etiology and pathogenesis. J Foot Surg 27,75, 1988 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials