Spontaneous recovery of fear reverses extinction-induced excitability of infralimbic neurons - PubMed (original) (raw)
Spontaneous recovery of fear reverses extinction-induced excitability of infralimbic neurons
Emmanuel Cruz et al. PLoS One. 2014.
Abstract
In rodents, the infralimbic (IL) region of the medial prefrontal cortex plays a key role in the recall of fear extinction. Previously we showed that fear conditioning decreases the intrinsic excitability of IL neurons, and that fear extinction reverses the depressed excitability. In the current study, we examined the time course of the extinction-induced changes in adolescent rats. Immediately after extinction, IL neurons continued to show depressed excitability. However 4 hours after extinction, IL neurons showed an increase in evoked spikes that correlated with a reduced fast afterhyperpolarizing potential. This suggests that acquisition of fear extinction induces an increase in spike firing 4 hours later, during the consolidation of extinction. We also examined IL excitability in a group of rats that showed spontaneous recovery of fear 17 days after extinction (SR group). Similar to neurons after fear conditioning, IL neurons from the SR group showed depressed intrinsic excitability compared to neurons 4 hours after extinction, suggesting that extinction-induced enhancement in intrinsic excitability decreases with time reverting back to a depressed state. These results suggest that plasticity in IL contributes to the spontaneous recovery of fear and preventing this depression of IL excitability could prolong fear extinction.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
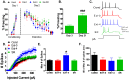
Figure 1. The intrinsic excitability of IL pyramidal neurons increased within 4 hours after extinction and reversed with the spontaneous recovery of fear.
A. Percent of time that rats spent freezing to the tone in groups that were conditioned (Cond; n = 13 rats, black), and fear extinguished (Ext-0; n = 9 rats, red; Ext-4; n = 6 rats, blue; SR; n = 8 rats, green). The Ext-0 group was sacrificed immediately after extinction, and the Ext-4 group was sacrificed 4 hours after extinction. B. Average freezing of the SR group on day 3 and day 21 to two tones. SR rats were sacrificed immediately after testing on day 21. C. Sample traces showing responses of IL neurons from each group to a 200 pA depolarizing current step. D. Number of spikes evoked in IL neurons by increasing depolarizing steps in the Cond (n = 34), Ext-0 (n = 22), Ext-4 (n = 18), and SR (n = 18) groups. E. Average maximum number of spikes evoked in each group. F. Average first interspike interval (ISI). ***p<0.001, *p<0.05.
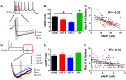
Figure 2. Spontaneous recovery reverses the decrease in fAHP induced by fear extinction.
A. Sample traces for each group showing fAHP measured within gray bar. Traces have been aligned to overlap at the spike threshold. B. Average of the fAHP for each group. C. Correlation between maximum number of spikes and fAHP for all neurons. D. Sample traces showing sAHP measured within gray bar. E. Average of the sAHP for each group. F. Lack of correlation between number of spikes and sAHP for all neurons. *p<0.05.
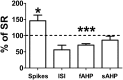
Figure 3. IL neurons of naïve age-matched rats are more excitable than IL neurons of the SR rats.
The number of spikes, ISI, fAHP, and sAHP of IL neurons (n = 15) of P50 naïve rats (n = 5) are shown normalized to the average values of the SR group. Dashed line indicates values of SR group.
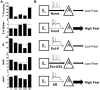
Figure 4. Summary and model of the changes in IL intrinsic excitability at different behavioral time-points.
A. Average values of the measured intrinsic parameters. C = COND, E0 = EXT-0, E4 = EXT-4, E24 = 24 hours after extinction, SR = Spontaneous Recovery. Dash-line indicates values of naïve group. Naïve and E24 values used with permission from the Journal of Neuroscience (Santini et al., 2008). B. Schematic representation of IL and amygdala interactions at different behavioral time-points.
Similar articles
- Muscarinic receptors modulate the intrinsic excitability of infralimbic neurons and consolidation of fear extinction.
Santini E, Sepulveda-Orengo M, Porter JT. Santini E, et al. Neuropsychopharmacology. 2012 Aug;37(9):2047-56. doi: 10.1038/npp.2012.52. Epub 2012 Apr 18. Neuropsychopharmacology. 2012. PMID: 22510723 Free PMC article. - Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons.
Santini E, Quirk GJ, Porter JT. Santini E, et al. J Neurosci. 2008 Apr 9;28(15):4028-36. doi: 10.1523/JNEUROSCI.2623-07.2008. J Neurosci. 2008. PMID: 18400902 Free PMC article. - M-type potassium channels modulate the intrinsic excitability of infralimbic neurons and regulate fear expression and extinction.
Santini E, Porter JT. Santini E, et al. J Neurosci. 2010 Sep 15;30(37):12379-86. doi: 10.1523/JNEUROSCI.1295-10.2010. J Neurosci. 2010. PMID: 20844133 Free PMC article. - Bidirectional changes in the intrinsic excitability of infralimbic neurons reflect a possible regulatory role in the acquisition and extinction of Pavlovian conditioned fear.
Rabinak CA, Zimmerman JM, Chang CH, Orsini CA. Rabinak CA, et al. J Neurosci. 2008 Jul 16;28(29):7245-7. doi: 10.1523/JNEUROSCI.2130-08.2008. J Neurosci. 2008. PMID: 18632927 Free PMC article. Review. No abstract available. - Learning-induced intrinsic and synaptic plasticity in the rodent medial prefrontal cortex.
Porter JT, Sepulveda-Orengo MT. Porter JT, et al. Neurobiol Learn Mem. 2020 Mar;169:107117. doi: 10.1016/j.nlm.2019.107117. Epub 2019 Nov 23. Neurobiol Learn Mem. 2020. PMID: 31765801 Free PMC article. Review.
Cited by
- Fear extinction requires infralimbic cortex projections to the basolateral amygdala.
Bloodgood DW, Sugam JA, Holmes A, Kash TL. Bloodgood DW, et al. Transl Psychiatry. 2018 Mar 6;8(1):60. doi: 10.1038/s41398-018-0106-x. Transl Psychiatry. 2018. PMID: 29507292 Free PMC article. - Neural Activity in Ventral Medial Prefrontal Cortex Is Modulated More Before Approach Than Avoidance During Reinforced and Extinction Trial Blocks.
Gentry RN, Roesch MR. Gentry RN, et al. J Neurosci. 2018 May 9;38(19):4584-4597. doi: 10.1523/JNEUROSCI.2579-17.2018. Epub 2018 Apr 16. J Neurosci. 2018. PMID: 29661965 Free PMC article. - Chronic nicotine exposure in preadolescence enhances later spontaneous recovery of fear memory.
Zeid D, Gould TJ. Zeid D, et al. Behav Neurosci. 2018 Aug;132(4):240-246. doi: 10.1037/bne0000247. Epub 2018 Jul 5. Behav Neurosci. 2018. PMID: 29975080 Free PMC article. - Contextual fear conditioning depresses infralimbic excitability.
Soler-Cedeño O, Cruz E, Criado-Marrero M, Porter JT. Soler-Cedeño O, et al. Neurobiol Learn Mem. 2016 Apr;130:77-82. doi: 10.1016/j.nlm.2016.01.015. Epub 2016 Feb 6. Neurobiol Learn Mem. 2016. PMID: 26860438 Free PMC article. - Fear Expression Suppresses Medial Prefrontal Cortical Firing in Rats.
Giustino TF, Fitzgerald PJ, Maren S. Giustino TF, et al. PLoS One. 2016 Oct 24;11(10):e0165256. doi: 10.1371/journal.pone.0165256. eCollection 2016. PLoS One. 2016. PMID: 27776157 Free PMC article.
References
- Bouton M, Westbrook R, Corcoran K, Maren S (2006) Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry 60 p. 352–360. - PubMed
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ (2007) Consolidation of Fear Extinction Requires NMDA Receptor-Dependent Bursting in the Ventromedial Prefrontal Cortex. Neuron 53(6) p. 871–80. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials