Molecular pathways: linking tumor microenvironment to epithelial-mesenchymal transition in metastasis - PubMed (original) (raw)
Review
Molecular pathways: linking tumor microenvironment to epithelial-mesenchymal transition in metastasis
Hae-Yun Jung et al. Clin Cancer Res. 2015.
Abstract
During tumor development, tumor cells constantly communicate with the surrounding microenvironment through both biochemical and biophysical cues. In particular, the tumor microenvironment can instruct carcinoma cells to undergo a morphogenesis program termed epithelial-to-mesenchymal transition (EMT) to facilitate local invasion and metastatic dissemination. Growing evidence uncovered a plethora of microenvironmental factors in promoting EMT, including proinflammatory cytokines secreted by locally activated stromal cells, hypoxia conditions, extracellular matrix components, and mechanical properties. Here, we review various biochemical and biophysical factors in the tumor microenvironment that directly impinge upon the EMT program. Specifically, cytokines such as TGFβ, TNFα, and IL6 and hypoxia are capable of inducing EMT in various tumors. Several extracellular matrix (ECM) proteins, including collagen-I, fibronectin, and hyaluronan, and ECM remodeling via extracellular lysyl oxidase are also implicated in regulating EMT. In preclinical studies and ongoing clinical trials, targeting these tumor microenvironmental signals has shown promises in halting tumor progression in various human cancers.
©2014 American Association for Cancer Research.
Figures
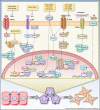
Figure 1. Regulation of EMT transcription factors by tumor microenvironmental signals
Transforming growth factor-β (TGFβ) regulates up-regulation of TWIST1, SNAIL1, and SNAIL2 via the SMAD signaling pathway. Drugs that inhibit TGFβ are AP 12009, GC1008, LY573636, which are in clinical trial for advanced solid tumors. Tumor necrosis factors-α (TNFα) activates NFκB to induce TWIST1, SNAIL2, and ZEB1/2 expression and TNFα/NFκB activation also increases SNAIL1 protein stability. Therapeutic approaches to inhibit TGFβ signaling include TNFα antagonist (infliximab and etanercept) and NFkB inhibitor (bortezomib), all of which have been assessed in phase II clinical trial for several cancer types. Interleukin-6 (IL6) induces TWIST1 and SNAIL1 expression via JAK/STAT3 signaling and increases TWIST1 stability through CK2-dependent phosphorylation. An IL6 ligand-blocking antibody, CNTO 328, has been tested in phase I/II clinical trials with metastatic renal cell carcinoma. Hypoxia inducible factor-1α (HIF1α) induces TWIST1 and SNAIL1 expression and HIF1α either alone or in cooperation with TGFβ promotes SNAIL1 nuclear localization to stabilize SNAIL. Agents to inhibit HIF1α include EZN-2698, PX-478, and topotecan. Topotecan has been tested in phase I/II clinical trials in combination with conventional chemotherapy and EZN-2698 and PX-478 are currently being tested in phase I clinical trial. Collagen I can promote SNAIL1 stability through binding to its receptor DDR2 and activating SRC/ERK2 pathway. Hyaluronan (HA) binding to CD44 induces nuclear translocation of CD44 to directly induce Lysyl-Oxidase (LOX) expression, which in turn increases TWIST1 expression.
Similar articles
- Regulation of epithelial-mesenchymal transition by tumor microenvironmental signals and its implication in cancer therapeutics.
Zhang J, Hu Z, Horta CA, Yang J. Zhang J, et al. Semin Cancer Biol. 2023 Jan;88:46-66. doi: 10.1016/j.semcancer.2022.12.002. Epub 2022 Dec 13. Semin Cancer Biol. 2023. PMID: 36521737 Free PMC article. Review. - Epithelial-mesenchymal transition and inflammation at the site of the primary tumor.
Dominguez C, David JM, Palena C. Dominguez C, et al. Semin Cancer Biol. 2017 Dec;47:177-184. doi: 10.1016/j.semcancer.2017.08.002. Epub 2017 Aug 18. Semin Cancer Biol. 2017. PMID: 28823497 Free PMC article. Review. - Targeting Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer.
Du B, Shim JS. Du B, et al. Molecules. 2016 Jul 22;21(7):965. doi: 10.3390/molecules21070965. Molecules. 2016. PMID: 27455225 Free PMC article. Review. - Tumor and its microenvironment: a synergistic interplay.
Catalano V, Turdo A, Di Franco S, Dieli F, Todaro M, Stassi G. Catalano V, et al. Semin Cancer Biol. 2013 Dec;23(6 Pt B):522-32. doi: 10.1016/j.semcancer.2013.08.007. Epub 2013 Sep 4. Semin Cancer Biol. 2013. PMID: 24012661 Review. - The tumor microenvironment: An irreplaceable element of tumor budding and epithelial-mesenchymal transition-mediated cancer metastasis.
Li H, Xu F, Li S, Zhong A, Meng X, Lai M. Li H, et al. Cell Adh Migr. 2016 Jul 3;10(4):434-46. doi: 10.1080/19336918.2015.1129481. Epub 2016 Jan 8. Cell Adh Migr. 2016. PMID: 26743180 Free PMC article. Review.
Cited by
- Overexpression of the oncostatin-M receptor in cervical squamous cell carcinoma is associated with epithelial-mesenchymal transition and poor overall survival.
Kucia-Tran JA, Tulkki V, Smith S, Scarpini CG, Hughes K, Araujo AM, Yan KY, Botthof J, Pérez-Gómez E, Quintanilla M, Cuschieri K, Caffarel MM, Coleman N. Kucia-Tran JA, et al. Br J Cancer. 2016 Jul 12;115(2):212-22. doi: 10.1038/bjc.2016.199. Epub 2016 Jun 28. Br J Cancer. 2016. PMID: 27351213 Free PMC article. - Paracrine factors from adipose-mesenchymal stem cells enhance metastatic capacity through Wnt signaling pathway in a colon cancer cell co-culture model.
Chen D, Liu S, Ma H, Liang X, Ma H, Yan X, Yang B, Wei J, Liu X. Chen D, et al. Cancer Cell Int. 2015 Apr 18;15:42. doi: 10.1186/s12935-015-0198-9. eCollection 2015. Cancer Cell Int. 2015. PMID: 26060426 Free PMC article. - Streptococcus mutans promotes tumor progression in oral squamous cell carcinoma.
Tsai MS, Chen YY, Chen WC, Chen MF. Tsai MS, et al. J Cancer. 2022 Sep 21;13(12):3358-3367. doi: 10.7150/jca.73310. eCollection 2022. J Cancer. 2022. PMID: 36186905 Free PMC article. - Sanguinarine inhibits epithelial-mesenchymal transition via targeting HIF-1α/TGF-β feed-forward loop in hepatocellular carcinoma.
Su Q, Fan M, Wang J, Ullah A, Ghauri MA, Dai B, Zhan Y, Zhang D, Zhang Y. Su Q, et al. Cell Death Dis. 2019 Dec 9;10(12):939. doi: 10.1038/s41419-019-2173-1. Cell Death Dis. 2019. PMID: 31819036 Free PMC article. - Macrophages and monocytes mediated activation of oxidative phosphorylation implicated the prognosis and clinical therapeutic strategy of Wilms tumour.
Meng J, Chen Y, Lu X, Ge Q, Yang F, Bai S, Liang C, Du J. Meng J, et al. Comput Struct Biotechnol J. 2022 Jun 27;20:3399-3408. doi: 10.1016/j.csbj.2022.06.052. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 35832632 Free PMC article.
References
- De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97–110. - PubMed
- Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. - PubMed
- Valdés F, Alvarez AM, Locascio A, Vega S, Herrera B, Fernández M, et al. The epithelial mesenchymal transition confers resistance to the apoptotic effects of transforming growth factor Beta in fetal rat hepatocytes. Mol Cancer Res. 2002;1:68–78. - PubMed
- Derksen PWB, Liu X, Saridin F, van der Gulden H, Zevenhoven J, Evers B, et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006;10:437–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources