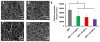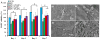Electrospun biodegradable elastic polyurethane scaffolds with dipyridamole release for small diameter vascular grafts - PubMed (original) (raw)
Electrospun biodegradable elastic polyurethane scaffolds with dipyridamole release for small diameter vascular grafts
Primana Punnakitikashem et al. Acta Biomater. 2014 Nov.
Abstract
Acellular biodegradable small diameter vascular grafts (SDVGs) require antithrombosis, intimal hyperplasia inhibition and rapid endothelialization to improve the graft patency. However, current antithrombosis and antiproliferation approaches often conflict with endothelial cell layer formation on SDVGs. To address this limitation, biodegradable elastic polyurethane urea (BPU) and the drug dipyridamole (DPA) were mixed and then electrospun into a biodegradable fibrous scaffold. The BPU would provide the appropriate mechanical support, while the DPA in the scaffold would offer biofunctions as required above. We found that the resulting scaffolds had tensile strengths and strains comparable with human coronary artery. The DPA in the scaffolds was continuously released up to 91 days in phosphate buffer solution at 37 °C, with a low burst release within the first 3 days. Compared to BPU alone, improved non-thrombogenicity of the DPA-loaded BPU scaffolds was evidenced with extended human blood clotting time, lower TAT complex concentration, lower hemolysis and reduced human platelet deposition. The scaffolds with a higher DPA content (5 and 10%) inhibited proliferation of human aortic smooth muscle cell significantly. Furthermore, the DPA-loaded scaffolds had no adverse effect on human aortic endothelial cell growth, yet it improved their proliferation. The attractive mechanical properties and biofunctions of the DPA-loaded BPU scaffold indicate its potential as an acellular biodegradable SDVG for vascular replacement.
Keywords: Controlled release; Dipyridamole; Electrospinning; Polyurethane; Small diameter vascular graft.
Copyright © 2014 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures
Fig. 1
Chemical structures of (A) BPU and (B) DPA.
Fig. 2
(A) A macroscopic view of a small diameter conduit from a mixture of BPU and 10% DPA to BPU by electrospinning. (B and C) Electronic cross-section images of the conduit.
Fig. 3
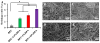
Micrographic fiber morphologies of (A) BPU, (B) BPU+2.5% DPA, (C) BPU+5% DPA and (D) BPU+10% DPA.
Fig. 4
(A) Shrinkage ratios of electrospun scaffolds and micrographic fiber morphologies of (B) BPU, (C) BPU+2.5% DPA, (D) BPU+5% DPA and (E) BPU+10% DPA after 24 h PBS immersion at 37°C.
Fig. 5
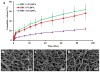
(A) In vitro DPA release curves of DPA-loaded BPU scaffolds up to 91 days in PBS at 37°C. SEM images exhibited fiber morphologies of (B) BPU+2.5% DPA, (C) BPU+5% DPA and (D) BPU+10% DPA after 91 day release.
Fig. 6
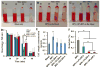
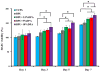
Digital images to show human blood clot formation with time of (A) human blood, (B) BPU alone in the human blood and (C) BPU+10% DPA in the human blood. (D) The absorbance at 540 nm of the lysate of human blood contacted with the scaffold. (E) The TAT complex concentration in PPP after the scaffold has been in contact with human blood. The control is human blood alone. (F) Human blood hemolysis percentages of the scaffolds.
Fig. 7
Human blood platelet depositions on the surfaces of (A) BPU, (B) BPU+2.5% DPA, (C) BPU+5% DPA and (D) BPU+10% DPA. (E) Quantification of human blood platelet deposition on the scaffold surfaces using an LDH assay.
Fig. 8
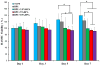
HASMC proliferation inhibition was evaluated using an MTT assay after incubating BPU and DPA-loaded BPU scaffolds in HASMC-preseeded wells. TCPS without any treatment was set as the control.
Fig. 9
Live/dead staining to show HASMC morphologies on (A) TCPS and after incubating (B) BPU, (C) BPU+2.5% DPA, (D) BPU+5% DPA and (E) BPU+10% DPA in HASMC-preseeded wells at day 7. Green: living cells; red: dead cells.
Fig. 10
HAEC proliferation was assessed using an MTT assay after placing BPU and DPA-loaded BPU scaffolds in HAEC-preseeded wells. TCPS without any treatment was used as the control.
Fig. 11
Live/dead staining to show HAEC morphologies on (A) TCPS and after incubating (B) BPU, (C) BPU+2.5% DPA, (D) BPU+5% DPA and (E) BPU+10% DPA in HAEC-preseeded wells at day 5. Green: living cells; red: dead cells.
Fig. 12
HAEC growth on the surfaces of BPU and DPA-loaded BPU scaffolds up to 7 days (A). The TCPS surface served as the control. SEM images showed HAEC morphology on the surfaces of (B) BPU, (C) BPU+2.5% DPA, (D) BPU+5% DPA and (E) BPU+10% DPA at day 7.
Similar articles
- Resveratrol-loaded polyurethane nanofibrous scaffold: viability of endothelial and smooth muscle cells.
Asadpour S, Yeganeh H, Khademi F, Ghanbari H, Ai J. Asadpour S, et al. Biomed Mater. 2019 Nov 15;15(1):015001. doi: 10.1088/1748-605X/ab4e23. Biomed Mater. 2019. PMID: 31618720 - In vitro physical and biological characterization of biodegradable elastic polyurethane containing ferulic acid for small-caliber vascular grafts.
Asadpour S, Ai J, Davoudi P, Ghorbani M, Jalali Monfared M, Ghanbari H. Asadpour S, et al. Biomed Mater. 2018 Mar 6;13(3):035007. doi: 10.1088/1748-605X/aaa8b6. Biomed Mater. 2018. PMID: 29345244 - Electrospun tecophilic/gelatin nanofibers with potential for small diameter blood vessel tissue engineering.
Vatankhah E, Prabhakaran MP, Semnani D, Razavi S, Morshed M, Ramakrishna S. Vatankhah E, et al. Biopolymers. 2014 Dec;101(12):1165-80. doi: 10.1002/bip.22524. Biopolymers. 2014. PMID: 25042000 - Quickening: Translational design of resorbable synthetic vascular grafts.
Stowell CET, Wang Y. Stowell CET, et al. Biomaterials. 2018 Aug;173:71-86. doi: 10.1016/j.biomaterials.2018.05.006. Epub 2018 May 5. Biomaterials. 2018. PMID: 29772461 Free PMC article. Review. - Small diameter vascular grafts: progress on electrospinning matrix/stem cell blending approach.
Wang N, Chen J, Hu Q, He Y, Shen P, Yang D, Wang H, Weng D, He Z. Wang N, et al. Front Bioeng Biotechnol. 2024 May 14;12:1385032. doi: 10.3389/fbioe.2024.1385032. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38807647 Free PMC article. Review.
Cited by
- Use of 3D Printing for the Development of Biodegradable Antiplatelet Materials for Cardiovascular Applications.
Domínguez-Robles J, Diaz-Gomez L, Utomo E, Shen T, Picco CJ, Alvarez-Lorenzo C, Concheiro A, Donnelly RF, Larrañeta E. Domínguez-Robles J, et al. Pharmaceuticals (Basel). 2021 Sep 11;14(9):921. doi: 10.3390/ph14090921. Pharmaceuticals (Basel). 2021. PMID: 34577621 Free PMC article. - PLLA-PHB fiber membranes obtained by solvent-free electrospinning for short-time drug delivery.
Cao K, Liu Y, Olkhov AA, Siracusa V, Iordanskii AL. Cao K, et al. Drug Deliv Transl Res. 2018 Feb;8(1):291-302. doi: 10.1007/s13346-017-0463-7. Drug Deliv Transl Res. 2018. PMID: 29235075 - Electrospun Nano-Fibers for Biomedical and Tissue Engineering Applications: A Comprehensive Review.
Parham S, Kharazi AZ, Bakhsheshi-Rad HR, Ghayour H, Ismail AF, Nur H, Berto F. Parham S, et al. Materials (Basel). 2020 May 6;13(9):2153. doi: 10.3390/ma13092153. Materials (Basel). 2020. PMID: 32384813 Free PMC article. Review. - Cilostazol-Loaded Poly(ε-Caprolactone) Electrospun Drug Delivery System for Cardiovascular Applications.
Rychter M, Baranowska-Korczyc A, Milanowski B, Jarek M, Maciejewska BM, Coy EL, Lulek J. Rychter M, et al. Pharm Res. 2018 Jan 16;35(2):32. doi: 10.1007/s11095-017-2314-0. Pharm Res. 2018. PMID: 29368067 Free PMC article. - Electrospun nanofiber scaffold for vascular tissue engineering.
Rickel AP, Deng X, Engebretson D, Hong Z. Rickel AP, et al. Mater Sci Eng C Mater Biol Appl. 2021 Oct;129:112373. doi: 10.1016/j.msec.2021.112373. Epub 2021 Aug 14. Mater Sci Eng C Mater Biol Appl. 2021. PMID: 34579892 Free PMC article. Review.
References
- Wang X, Lin P, Yao Q, Chen C. Development of small-diameter vascular grafts. World J Surg. 2007;31:682–9. - PubMed
- Koch S, Flanagan TC, Sachweh JS, Tanios F, Schnoering H, Deichmann T, et al. Fibrin–polylactide-based tissue-engineered vascular graft in the arterial circulation. Biomaterials. 2010;31:4731–9. - PubMed
- Bailey SR, Polan JL, Munoz OC, Agrawal MC, Goswami NJ. Proliferation and I2-tubulin for human aortic endothelial cells within gas–plasma scaffolds. Cardiovasc Radiat Med. 2004;5:119–24. - PubMed
- Gao J, Crapo P, Nerem R, Wang Y. Co-expression of elastin and collagen leads to highly compliant engineered blood vessels. J Biomed Mater Res Part A. 2008;85A:1120–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources