miRNA expression in anaplastic thyroid carcinomas - PubMed (original) (raw)
miRNA expression in anaplastic thyroid carcinomas
Aline Hébrant et al. PLoS One. 2014.
Abstract
Anaplastic thyroid carcinoma (ATC) is the most lethal form of thyroid neoplasia and represents an end stage of thyroid tumor progression. No effective treatment exists so far. In this study, we analyzed the miRNA expression profiles of 11 ATC by microarrays and their relationship with the mRNA expression profiles of the same 11 ATC samples. ATC show distinct miRNA expression profiles compared to other less aggressive thyroid tumor types. ATC show 18 commonly deregulated miRNA compared to normal thyroid tissue (17 downregulated and 1 upregulated miRNA). First, the analysis of a combined approach of the mRNA gene expression and of the bioinformatically predicted mRNA targets of the deregulated miRNA suggested a role for these regulations in the epithelial to mesenchymal transition (EMT) process in ATC. Second, the direct interaction between one of the upregulated mRNA target, the LOX gene which is an EMT key player, and a downregulated miRNA, the miR-29a, was experimentally validated by a luciferase assay in HEK cell. Third, we confirmed that the ATC tissue is composed of about 50% of tumor associated macrophages (TAM) and suggested, by taking into account our data and published data, their most likely direct or paracrine intercommunication between them and the thyroid tumor cells, amplifying the tumor aggressiveness. Finally, we demonstrated by in situ hybridization a specific thyrocyte localization of 3 of the deregulated miRNA: let-7g, miR-29a and miR-30e and we pointed out the importance of identifying the cell type localization before drawing any conclusion on the physiopathological role of a given gene.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
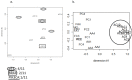
Figure 1. Multidimensional scaling (MDS) of the miRNA expression data: a. from 11 ATC (the ATC are labeled according to their mutation as depicted in the figure), b. from 11 ATC and 5 PTC (PC), 5 AA (AA), 5 FTC (FC) and 4 FTA (FA).
All human miRNA present on the arrays were considered for the analyses.
Figure 2. Immunohistochemistry with the CD163 antibody on 3 different ATC and on normal thyroid tissue.
a, b, c: 20× magnification showing that about 50% of the nucleated cells are TAM, d: 40× magnification showing elongated cytoplasmic extensions in TAM as illustrated by the red arrows; e: 20× magnification illustrating the almost absence of TAM in normal thyroid tissue.
Figure 3. In situ hybridization of Let-7g, miR-29a and miR-30e in normal and ATC tissues.
Tissue sections from normal and tumor thyroids were incubated with a full length DIG-labeled LNA probe to detect Let-7g, miR-29a and miR-30e: 20× magnification showing a strong miRNA signal observed (in blue) in the normal thyrocytes and a weak signal observed in the tumor cells derived from thyrocytes. No signal was detected in the TAM. 4 ATC were investigated. Representative pictures of 3 of them are shown.
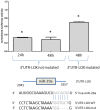
Figure 4. Normalized luciferase activity in HEK293 cells cotransfected during 24 h or 48 h with the 3′UTR-LOX construction or with the mutated construction (3′UTR-LOX mutated: partial deletion of the seed sequence) and with miR-29a or with the scramble.
The seed sequence is represented in blue and the * indicates a p-value<5%.
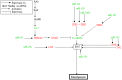
Figure 5. Proposed scheme synthetizing the miRNA and mRNA expression analyses of the same 11 ATC and highlighting the EMT feature of this tumor.
Only direct binding of miRNA on mRNA are represented (green: downregulated m(i)RNA; red: upregulated m(i)RNA.
Similar articles
- MicroRNA 483-3p targets Pard3 to potentiate TGF-β1-induced cell migration, invasion, and epithelial-mesenchymal transition in anaplastic thyroid cancer cells.
Zhang X, Liu L, Deng X, Li D, Cai H, Ma Y, Jia C, Wu B, Fan Y, Lv Z. Zhang X, et al. Oncogene. 2019 Jan;38(5):699-715. doi: 10.1038/s41388-018-0447-1. Epub 2018 Aug 31. Oncogene. 2019. PMID: 30171257 Free PMC article. - miR-199a-5p suppresses epithelial- mesenchymal-transition in anaplastic thyroid carcinoma cells via targeting Snail signals.
Hao F, Bi YN, Wang L, Wang Y, Ma J, Cui P, Li X, Sun S, Ning L, Huang Y, Jiao X, Chen D. Hao F, et al. Cancer Biomark. 2020;29(3):317-326. doi: 10.3233/CBM-201518. Cancer Biomark. 2020. PMID: 32716347 Retracted. - Transcript-level regulation of MALAT1-mediated cell cycle and apoptosis genes using dual MEK/Aurora kinase inhibitor "BI-847325" on anaplastic thyroid carcinoma.
Samimi H, Haghpanah V, Irani S, Arefian E, Sohi AN, Fallah P, Soleimani M. Samimi H, et al. Daru. 2019 Jun;27(1):1-7. doi: 10.1007/s40199-018-0231-3. Epub 2019 May 10. Daru. 2019. PMID: 31077090 Free PMC article. - Expression of MicroRNAs in Thyroid Carcinoma.
Zhu G, Xie L, Miller D. Zhu G, et al. Methods Mol Biol. 2017;1617:261-280. doi: 10.1007/978-1-4939-7046-9_19. Methods Mol Biol. 2017. PMID: 28540691 Review. - Role of microRNAs in endocrine cancer metastasis.
Lima CR, Gomes CC, Santos MF. Lima CR, et al. Mol Cell Endocrinol. 2017 Nov 15;456:62-75. doi: 10.1016/j.mce.2017.03.015. Epub 2017 Mar 18. Mol Cell Endocrinol. 2017. PMID: 28322989 Review.
Cited by
- Expression profiles of pivotal microRNAs and targets in thyroid papillary carcinoma: an analysis of The Cancer Genome Atlas.
Cong D, He M, Chen S, Liu X, Liu X, Sun H. Cong D, et al. Onco Targets Ther. 2015 Aug 26;8:2271-7. doi: 10.2147/OTT.S85753. eCollection 2015. Onco Targets Ther. 2015. PMID: 26345235 Free PMC article. - Lysyl Oxidase and the Tumor Microenvironment.
Wang TH, Hsia SM, Shieh TM. Wang TH, et al. Int J Mol Sci. 2016 Dec 29;18(1):62. doi: 10.3390/ijms18010062. Int J Mol Sci. 2016. PMID: 28036074 Free PMC article. Review. - MiRNA Deregulation Distinguishes Anaplastic Thyroid Carcinoma (ATC) and Supports Upregulation of Oncogene Expression.
Misiak D, Bauer M, Lange J, Haase J, Braun J, Lorenz K, Wickenhauser C, Hüttelmaier S. Misiak D, et al. Cancers (Basel). 2021 Nov 24;13(23):5913. doi: 10.3390/cancers13235913. Cancers (Basel). 2021. PMID: 34885022 Free PMC article. - Inflammatory Components of the Thyroid Cancer Microenvironment: An Avenue for Identification of Novel Biomarkers.
Jarboe T, Tuli NY, Chakraborty S, Maniyar RR, DeSouza N, Xiu-Min Li, Moscatello A, Geliebter J, Tiwari RK. Jarboe T, et al. Adv Exp Med Biol. 2021;1350:1-31. doi: 10.1007/978-3-030-83282-7_1. Adv Exp Med Biol. 2021. PMID: 34888842 - Poorly Differentiated and Anaplastic Thyroid Cancer: Insights into Genomics, Microenvironment and New Drugs.
Prete A, Matrone A, Gambale C, Torregrossa L, Minaldi E, Romei C, Ciampi R, Molinaro E, Elisei R. Prete A, et al. Cancers (Basel). 2021 Jun 26;13(13):3200. doi: 10.3390/cancers13133200. Cancers (Basel). 2021. PMID: 34206867 Free PMC article. Review.
References
- Ain KB (1999) Anaplastic thyroid carcinoma: a therapeutic challenge. Semin Surg Oncol 16: 64–69. - PubMed
- Are C, Shaha AR (2006) Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol 13: 453–464. - PubMed
- Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. (2006) Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 16: 109–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was supported by Welbio, Télévie, European Union (GENRISK-T project 036495), Fonds de la Recherche Scientifique Médicale, Fondation Van Buren, Les amis de l'Institut Bordet, Fondation Contre le Cancer, Fonds National de la Recherche Scientifique (FNRS). ATC tissue samples from Lille were obtained from the tumour cell and tissue bank of Regional Reference Cancer Center of Lille “Tumorothèque du centre régional de Référence en Cancérologie” (France). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous