State-of-the-art human gene therapy: part II. Gene therapy strategies and clinical applications - PubMed (original) (raw)
Review
. 2014 Sep;18(98):151-61.
Affiliations
- PMID: 25227756
- PMCID: PMC4440458
Review
State-of-the-art human gene therapy: part II. Gene therapy strategies and clinical applications
Dan Wang et al. Discov Med. 2014 Sep.
Abstract
In Part I of this Review (Wang and Gao, 2014), we introduced recent advances in gene delivery technologies and explained how they have powered some of the current human gene therapy applications. In Part II, we expand the discussion on gene therapy applications, focusing on some of the most exciting clinical uses. To help readers to grasp the essence and to better organize the diverse applications, we categorize them under four gene therapy strategies: (1) gene replacement therapy for monogenic diseases, (2) gene addition for complex disorders and infectious diseases, (3) gene expression alteration targeting RNA, and (4) gene editing to introduce targeted changes in host genome. Human gene therapy started with the simple idea that replacing a faulty gene with a functional copy can cure a disease. It has been a long and bumpy road to finally translate this seemingly straightforward concept into reality. As many disease mechanisms unraveled, gene therapists have employed a gene addition strategy backed by a deep knowledge of what goes wrong in diseases and how to harness host cellular machinery to battle against diseases. Breakthroughs in other biotechnologies, such as RNA interference and genome editing by chimeric nucleases, have the potential to be integrated into gene therapy. Although clinical trials utilizing these new technologies are currently sparse, these innovations are expected to greatly broaden the scope of gene therapy in the near future.
Figures
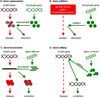
Figure 1
Depiction of four gene therapy strategies. (A) A gene mutation (red dot) abrogates protein synthesis (red cross) and leads to disease. Gene replacement corrects the disease by providing a functional copy of the gene (green helix) and normal proteins (green circles). (B) Multiple genetic factors and/or environmental factors can result in a disease though a complex disease cascade (dashed arrow). Gene addition alleviates the disease by supplementing therapeutic genes that target a specific aspect of the disease mechanism. (C) A gene mutation leads to the production of toxic protein aggregate (clustered red circles) that causes a disease. Gene knockdown utilizes small RNAs (green combs) to inhibit the aberrant mRNA (black comb), thus preventing the synthesis of toxic protein aggregate and correcting the disease. (D) Gene editing by chimeric nucleases (circle and pie) is a versatile approach to make a targeted change from a disease-promoting sequence (red dot) to a disease-preventing sequence (green dot). Also see Figure 4.
Figure 2
Breakdown of approved gene therapy clinical trials worldwide since 1989 according to disease applications. Data are from Wiley Gene Therapy Clinical Trials Worldwide (
).
Figure 3
Reprogramming mRNA splicing by AONs. (A) Exon skipping to restore dystrophin in DMD. Normal splicing of DMD pre-mRNA leads to mature mRNA consisting of all exons that direct the synthesis of full-length dystrophin. In DMD, mutations in exon 50 result in abnormal splicing that excludes exon 50 in mature mRNA, which shifts reading frame and abrogates dystrophin production. AON binding blocks splicing signal in exon 51, which excludes exon 51 together with exon 50, thus restoring original reading frame and producing partially functional, albeit shortened, dystrophin. (B) Exon inclusion to restore SMN protein in SMA. Under normal condition, only the SMN1 gene produces SMN protein, whereas SMN2 gene is largely silenced due to a single nucleotide change in exon 7 (yellow bar), which excludes exon 7 in mature mRNA. In SMA, SMN1 is mutated (e.g. gene deletion), resulting in loss of SMN protein. A bi-functional AON binds to exon 7 of SMN2 pre-mRNA and recruits splicing factor (SF) that mediates the splicing pattern as in normal SMN1 pre-mRNA. Therefore, the silenced SMN2 gene is reactivated and provides therapeutic SMN proteins. Boxes: exons. Curved arrows: protein synthesis. Black stop signs: normal stop codons. Red stop sign: premature stop codon caused by frameshifting. Green stem loops: RNA AON embedded in modified U7 small nuclear RNA. DSB: double-strand break. DMD: Duchene muscular dystrophy. AON: antisense oligonucleotide. SMA: spinal muscular atrophy. SMN: survival of motor neuron.
Figure 4
Gene editing by ZFN to introduce targeted changes in host genome. (A) Gene disruption through NHEJ. ZFN-induced DSB (white gap) in a disease-promoting gene (black box) is repaired through random insertion/deletion of a few nucleotides (red gap), which diminishes the disease-promoting function of the gene (red cross). (B) In the presence of a donor template with homology to the targeted genomic region, ZFN-induced DSB triggers homology-mediated recombination (dashed crosses), so that the therapeutic gene carried in the donor template is added to the host genome. If the DSB is targeted to a mutated gene region, HR can replace the faulty gene with a normal copy in the donor template. Black boxes: genes in host genome. Green boxes: therapeutic genes carried in donor templates. NHEJ: nonhomologous end joining. ZFN: zinc-finger nuclease. DSB: double-strand break. HR: homologous recombination.
Similar articles
- Progress toward therapy with antisense-mediated splicing modulation.
Du L, Gatti RA. Du L, et al. Curr Opin Mol Ther. 2009 Apr;11(2):116-23. Curr Opin Mol Ther. 2009. PMID: 19330717 Free PMC article. Review. - Modulating the expression of disease genes with RNA-based therapy.
Wood M, Yin H, McClorey G. Wood M, et al. PLoS Genet. 2007 Jun;3(6):e109. doi: 10.1371/journal.pgen.0030109. PLoS Genet. 2007. PMID: 17604456 Free PMC article. Review. - Gene Therapy with CRISPR/Cas9 Coming to Age for HIV Cure.
Soriano V. Soriano V. AIDS Rev. 2017 Oct-Dec;19(3):167-172. AIDS Rev. 2017. PMID: 29019352 - Therapeutic Modulation of RNA Splicing in Malignant and Non-Malignant Disease.
El Marabti E, Abdel-Wahab O. El Marabti E, et al. Trends Mol Med. 2021 Jul;27(7):643-659. doi: 10.1016/j.molmed.2021.04.005. Epub 2021 May 13. Trends Mol Med. 2021. PMID: 33994320 Free PMC article. Review. - Current Progress in Therapeutic Gene Editing for Monogenic Diseases.
Prakash V, Moore M, Yáñez-Muñoz RJ. Prakash V, et al. Mol Ther. 2016 Mar;24(3):465-74. doi: 10.1038/mt.2016.5. Epub 2016 Jan 14. Mol Ther. 2016. PMID: 26765770 Free PMC article. Review.
Cited by
- Gene Therapy Options as New Treatment for Inherited Peripheral Neuropathy.
Thenmozhi R, Lee JS, Park NY, Choi BO, Hong YB. Thenmozhi R, et al. Exp Neurobiol. 2020 Jun 30;29(3):177-188. doi: 10.5607/en20004. Exp Neurobiol. 2020. PMID: 32624504 Free PMC article. Review. - The Potential of Induced Pluripotent Stem Cells to Test Gene Therapy Approaches for Neuromuscular and Motor Neuron Disorders.
Cappella M, Elouej S, Biferi MG. Cappella M, et al. Front Cell Dev Biol. 2021 Apr 13;9:662837. doi: 10.3389/fcell.2021.662837. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33937264 Free PMC article. Review. - Current Landscape of Gene Therapy for the Treatment of Cardiovascular Disorders.
Wal P, Aziz N, Singh CP, Rasheed A, Tyagi LK, Agrawal A, Wal A. Wal P, et al. Curr Gene Ther. 2024;24(5):356-376. doi: 10.2174/0115665232268840231222035423. Curr Gene Ther. 2024. PMID: 38288826 Review. - Current Therapeutic Approaches in Leukodystrophies: A Review.
Gordon-Lipkin E, Fatemi A. Gordon-Lipkin E, et al. J Child Neurol. 2018 Nov;33(13):861-868. doi: 10.1177/0883073818792313. Epub 2018 Aug 16. J Child Neurol. 2018. PMID: 30112967 Free PMC article. Review. - In vivo Antitumor Effect of an HPV-specific Promoter driving IL-12 Expression in an HPV 16-positive Murine Model of Cervical Cancer.
Bermúdez-Morales VH, Fierros-Zarate G, García-Meléndrez C, Alcocer-Gonzalez JM, Morales-Ortega A, Peralta-Zaragoza O, Torres-Poveda K, Burguete-García AI, Hernández-Márquez E, Madrid-Marina V. Bermúdez-Morales VH, et al. J Cancer. 2016 Sep 27;7(14):1950-1959. doi: 10.7150/jca.15536. eCollection 2016. J Cancer. 2016. PMID: 27877210 Free PMC article.
References
- Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L, Scaramuzza S, Andolfi G, Mirolo M, Brigida I, Tabucchi A, Carlucci F, Eibl M, Aker M, Slavin S, Al-Mousa H, Al Ghonaium A, Ferster A, Duppenthaler A, Notarangelo L, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360(5):447–458. - PubMed
- Andtbacka RH, Ross MI, Delman K, Noyes D, Zager JS, Hsueh E, Ollila DW, Amatruda T, Chen L, Kaufman H. Society of Surgical Oncology Cancer Symposium. Phoenix, Arizona: 2014. Responses of Injected and Uninjected Lesions to Intralesional Talimogene Laherparepvec (T-VEC) in the OPTiM Study and the Contribution of Surgery to Response.
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358(21):2231–2239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 DA031952/DA/NIDA NIH HHS/United States
- 1R01NS076991-01/NS/NINDS NIH HHS/United States
- P01 AI100263/AI/NIAID NIH HHS/United States
- P01 HL059407-11/HL/NHLBI NIH HHS/United States
- R01 NS076991/NS/NINDS NIH HHS/United States
- P01 HL059407/HL/NHLBI NIH HHS/United States
- P01 AI100263-01/AI/NIAID NIH HHS/United States
- 1R21DA031952-01A/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials