miR-17-92 cluster promotes cholangiocarcinoma growth: evidence for PTEN as downstream target and IL-6/Stat3 as upstream activator - PubMed (original) (raw)
miR-17-92 cluster promotes cholangiocarcinoma growth: evidence for PTEN as downstream target and IL-6/Stat3 as upstream activator
Hanqing Zhu et al. Am J Pathol. 2014 Oct.
Abstract
miR-17-92 is an oncogenic miRNA cluster implicated in the development of several cancers; however, it remains unknown whether the miR-17-92 cluster is able to regulate cholangiocarcinogenesis. This study was designed to investigate the biological functions and molecular mechanisms of the miR-17-92 cluster in cholangiocarcinoma. In situ hybridization and quantitative RT-PCR analysis showed that the miR-17-92 cluster is highly expressed in human cholangiocarcinoma cells compared with the nonneoplastic biliary epithelial cells. Forced overexpression of the miR-17-92 cluster or its members, miR-92a and miR-19a, in cultured human cholangiocarcinoma cells enhanced tumor cell proliferation, colony formation, and invasiveness, in vitro. Overexpression of the miR-17-92 cluster or miR-92a also enhanced cholangiocarcinoma growth in vivo in hairless outbred mice with severe combined immunodeficiency (SHO-Prkdc(scid)Hr(hr)). The tumor-suppressor, phosphatase and tensin homolog deleted on chromosome 10 (PTEN), was identified as a bona fide target of both miR-92a and miR-19a in cholangiocarcinoma cells via sequence prediction, 3' untranslated region luciferase activity assay, and Western blot analysis. Accordingly, overexpression of the PTEN open reading frame protein (devoid of 3' untranslated region) prevented miR-92a- or miR-19a-induced cholangiocarcinoma cell growth. Microarray analysis revealed additional targets of the miR-17-92 cluster in human cholangiocarcinoma cells, including APAF-1 and PRDM2. Moreover, we observed that the expression of the miR-17-92 cluster is regulated by IL-6/Stat3, a key oncogenic signaling pathway pivotal in cholangiocarcinogenesis. Taken together, our findings disclose a novel IL-6/Stat3-miR-17-92 cluster-PTEN signaling axis that is crucial for cholangiocarcinogenesis and tumor progression.
Copyright © 2014 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
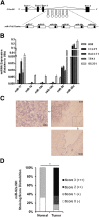
High expression of the miR-17-92 cluster in human cholangiocarcinoma tissues and cell lines. A: Genomic structure of the miR-17-92 cluster. The miR-17-92 cluster is polycistronic, and located at C13orf25. The pre–miR-17-92 cluster is cleaved, generating six mature miRNAs (miR-17, miR-18, miR-19a, miR-20, miR-19b, and miR-92). B: Expression of the miR-17-92 cluster in cholangiocarcinoma cell lines. RT-qPCR for mature miRNA in the miR-17-92 cluster in a human transformed cholangiocyte cell line (H69) and four human cholangiocarcinoma cell lines (CCLP1, HuCCT1, SG231, and TFK1). Total cellular RNA was extracted for RT-qPCR (n = 3). The expression level of each individual miRNA is normalized to RNU6B (internal control). All of the six miRNAs (miR-17/18/19a/19b/20/92) are highly expressed in four cholangiocarcinoma cell lines (CCLP-1, HuCCT1, SG231, and TFK-1 cells) compared with the noncancerous control cell line H69. Among the six mature miRNAs, miR-92a is the most abundant, followed by miR-17 and miR-20. C:In situ hybridization for mature miR-92a in human cholangiocarcinoma tissues. Representative in situ hybridization images depict the expression of miR-92a in human cholangiocarcinoma tissues. Note positive miR-92a staining in cholangiocarcinoma cells and negative staining in normal bile duct epithelial cells (positive signals are in dark blue; nuclei were counterstained in red). D: Distribution of miR-92a staining intensity scores in human cholangiocarcinoma tissue arrays (N = 44). ∗P < 0.05 (Kruskal-Wallis nonparameter test).
Figure 2
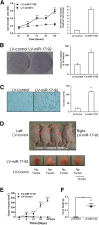
miR-92a overexpression promotes cholangiocarcinoma cell growth in vitro and in vivo. A–C: miR-92a overexpression promotes cholangiocarcinoma cell growth, colony formation, and cell invasion in vitro. Human cholangiocarcinoma cells (CCLP1) were infected with miR-92a lentivirus and miRNA scrambled control lentivirus, respectively. The stably transduced cells were analyzed for proliferation, colonogenic potential, and invasion ability, as described in Materials and Methods. A: miR-92a overexpression promotes cholangiocarcinoma cell growth. Cell growth curves of human cholangiocarcinoma cells (CCLP1) stably transduced with miR-92a lentivirus (indicated as LV-miR-92a) or scrambled control (indicated as LV-control). miR-92a levels were measured by RT-qPCR. B: miR-92a overexpression enhances cholangiocarcinoma cell colony formation. The numbers of colonies were counted after 14 days. Representative images showing colonies formed in cell culture dishes. Average colony formation efficiency is shown. C: miR-92a overexpression promotes cholangiocarcinoma cell invasion. Representative images showing migrated cell staining by hematoxylin. Average numbers of the invaded cells are shown. D–G: miR-92a promotes cholangiocarcinoma growth in vivo. miR-92a–overexpressed or scrambled control CCLP1 cells (1.0 × 108) were inoculated s.c. into flank areas of SCID hairless mice (n = 6), and the mice were closely monitored for tumor growth. Thirty days after inoculation, the mice were sacrificed and the tumors were recovered. D: Photographs of xenograft tumor masses from SCID mice highlight that miR-92a promotes tumor growth. Images are of the mice and the xenograft tumors that were recovered. E: miR-92a overexpression significantly increases xenograft tumor volume. F: miR-92a significantly increases the weight of the xenograft tumors. G: Western blot analysis reveals decreased PTEN protein levels in xenograft tumor tissues by miR-92a. Data are presented as means ± SEM (A–C) or means ± SD (E and F). N = 3 (A–C); N = 6 (E and F). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Figure 3
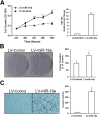
Overexpression of miR-17-92 cluster promotes cholangiocarcinoma cell growth in vitro and in vivo. The miR-17-92 cluster promotes human cholangiocarcinoma cell proliferation (A), colony formation (B), and cell invasion (C) in vitro. Human cholangiocarcinoma cells (CCLP1) were infected with miR-17-92 cluster lentivirus and miRNA scrambled control lentivirus, respectively, and the stable cells were analyzed for proliferation, colonogenic potential, and invasion ability, as described in Materials and Methods. A: Cell proliferation assay (WST-1). Cell growth curves of human cholangiocarcinoma cells (CCLP1) stably transduced with miR-17-92 cluster lentivirus (indicated as LV-miR-17-92) or scrambled control LV-control. The miR-17-92 cluster promotes cell growth in cholangiocarcinoma cell lines. Pre–miR-17-92 cluster levels were measured by RT-qPCR. B: miR-17-92 cluster overexpression enhances cholangiocarcinoma cell colony formation. The numbers of colonies were counted after 14 days. Representative images show colonies formed in cell culture dishes. Average colony-forming efficiency is shown. C: miR-17-92 cluster overexpression promotes cholangiocarcinoma cell invasion. Representative images show migrated cell staining by hematoxylin. Average numbers of invaded cells are shown. D–F: The miR-17-92 cluster promotes cholangiocarcinoma growth in vivo. miR-17-92 cluster–overexpressed or scrambled control CCLP1 cells (1.0 × 108) were inoculated s.c. into flank areas of SCID hairless mice (n = 6), and the mice were closely monitored for tumor growth. Thirty days after inoculation, the mice were sacrificed and the tumors were recovered. D: Photographs of xenograft tumor masses from SCID mice show that the miR-17-92 cluster promotes tumor growth. E: The volume of xenograft tumors is significantly increased by miR-17-92 cluster overexpression. F: The weight of xenograft tumors is significantly increased by miR-17-92 cluster overexpression. Data are from four SCID mice (two mice died before 30 days). Data represent means ± SEM (A–C) or means ± SD (E and F). N = 3 (A–C); N = 4 to 6 (E); N = 4 (F). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Figure 4
miR-19a overexpression promotes cholangiocarcinoma cell growth and colony formation in vitro. A: miR-19a overexpression promotes cholangiocarcinoma cell growth. Cell growth curves of human cholangiocarcinoma cells (CCLP1) stably transduced with miR-19a lentivirus (indicated as LV-miR-19a) or scrambled control (indicated as LV-control). miR-19a levels were measured by RT-qPCR. B: miR-19a overexpression promotes cholangiocarcinoma cell colony formation. The numbers of colonies were counted after 14 days. Representative images show colonies formed in cell culture dishes. Average colony formation efficiency is shown. C: miR-19a overexpression promotes cholangiocarcinoma cell invasion. Representative images show migrated cell staining by hematoxylin. Average numbers of invaded cells are shown. Data are presented as means ± SEM. N = 3. ∗P < 0.05, ∗∗∗P < 0.001.
Figure 5
miR-17-92 targets PTEN via miR-92a and miR-19a in cholangiocarcinoma cells. A: Predicted 8-mer and 7-mer-m8 binding sequence for miR-92a and miR-19a in the 3′UTR of PTEN mRNA. B: PTEN mRNA levels are decreased in miR-19a, miR-92a, or miR-17-92 cluster–overexpressed cells, as determined by RT-qPCR analysis. C: PTEN protein levels are down-regulated in miR-19a, miR-92a, and miR-17-92 cluster–overexpressed stable cell lines, as determined by using Western blot analysis. PTEN protein levels are also down-regulated by miR-19a mimic or miR-92a mimic in CCLP-1 cells. D: miR-92a or miR-19a mimic significantly reduces PTEN 3′UTR luciferase activity. CCLP1 cells were transfected with miRNA mimic or scrambled control, together with PTEN 3′UTR dual-luciferase reporter plasmid. The luciferase activity was analyzed 24 hours after transfection. E: PTEN (ORF) overexpression inhibits the cell growth induced by miR-19a or miR-92a. Data are presented as means ± SEM (B and E) or means ± SD (D). N = 3 (B and D); N = 6 (E). ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001.
Figure 6
IL-6 up-regulates miR-17-92 expression in cholangiocarcinoma cells through Stat3 activation. A: Stimulation of CCLP-1 cells with 20 ng/mL IL-6 increases the expression of the pre–miR-17-92 cluster, as determined by RT-qPCR. B: IL-6–mediated induction of the pre–miR-17-92 cluster is abolished by siRNA knockdown of Stat3 or the IL-6 receptor, GP130. RT-qPCR data are presented. C: IL-6–mediated induction of the pre–miR-17-92 cluster is abolished by 20 μmol/L Stat3 phosphorylation inhibitor Stattic. RT-qPCR data are presented. D: IL-6–mediated induction of the pre–miR-17-92 cluster is abrogated by 20 μmol/L JAK inhibitor, AZD1480 or INCB18424. RT-qPCR data are presented. E: IL-6 treatment increases binding of activated Stat3 to the promoter region of the miR-17-92 cluster, as determined by biotinylated oligonucleotide precipitation assay. Stat3 binding sequence at promoter region of pre–miR-17-92 was biotinylated-linked and used to precipitate proteins after 20 ng/mL IL-6 treatment for 1 hour. Phospho-Stat3 protein was detected by using Western blot analysis. F: Chromatin immunoprecipitation assay. The chromatin extracted from CCLP1 cells, treated with IL-6 or control vehicle, was subjected to immunoprecipitation with phospho-STAT3 antibody, and the precipitates were subjected to RT-qPCR analysis using primers to amplify the STAT3 binding region. Normal rabbit IgG was used as the negative control. RT-qPCR data are presented. Data are presented as means ± SEM (A–D and F). N = 3 (A–D and F). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Figure 7
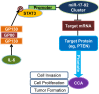
Schematic illustration of the proposed mechanism for miR-17-92 cluster–induced cholangiocarcinoma growth. The miR-17-92 cluster can directly target PTEN via miR-19a and miR-92, which associate with 3′UTR of PTEN. Reduction of PTEN enhances cholangiocarcinoma cell proliferation and invasion in vitro and tumor formation in vivo. Furthermore, IL-6 can up-regulate the expression of miR-17-92 cluster through Stat3-mediated transcription of the pre–miR-17-92 cluster. CCA, cholangiocarcinoma.
Similar articles
- Microsomal prostaglandin E synthase-1 inhibits PTEN and promotes experimental cholangiocarcinogenesis and tumor progression.
Lu D, Han C, Wu T. Lu D, et al. Gastroenterology. 2011 Jun;140(7):2084-94. doi: 10.1053/j.gastro.2011.02.056. Epub 2011 Feb 24. Gastroenterology. 2011. PMID: 21354147 Free PMC article. - PTEN and PDCD4 are bona fide targets of microRNA-21 in human cholangiocarcinoma.
Liu CZ, Liu W, Zheng Y, Su JM, Li JJ, Yu L, He XD, Chen SS. Liu CZ, et al. Chin Med Sci J. 2012 Jun;27(2):65-72. Chin Med Sci J. 2012. PMID: 22770403 - miR-21 targets 15-PGDH and promotes cholangiocarcinoma growth.
Lu L, Byrnes K, Han C, Wang Y, Wu T. Lu L, et al. Mol Cancer Res. 2014 Jun;12(6):890-900. doi: 10.1158/1541-7786.MCR-13-0419. Epub 2014 Apr 3. Mol Cancer Res. 2014. PMID: 24699315 Free PMC article. - MicroRNA-92a promotes epithelial-mesenchymal transition through activation of PTEN/PI3K/AKT signaling pathway in non-small cell lung cancer metastasis.
Lu C, Shan Z, Hong J, Yang L. Lu C, et al. Int J Oncol. 2017 Jul;51(1):235-244. doi: 10.3892/ijo.2017.3999. Epub 2017 May 16. Int J Oncol. 2017. PMID: 28534966 - Insights into the role of STAT3 in intrahepatic cholangiocarcinoma (Review).
Yang R, Song Y, Shakoor K, Yi W, Peng C, Liu S. Yang R, et al. Mol Med Rep. 2022 May;25(5):171. doi: 10.3892/mmr.2022.12687. Epub 2022 Mar 18. Mol Med Rep. 2022. PMID: 35302174 Free PMC article. Review.
Cited by
- Melanoma Brain Metastasis: Mechanisms, Models, and Medicine.
Kircher DA, Silvis MR, Cho JH, Holmen SL. Kircher DA, et al. Int J Mol Sci. 2016 Sep 2;17(9):1468. doi: 10.3390/ijms17091468. Int J Mol Sci. 2016. PMID: 27598148 Free PMC article. Review. - MicroRNA-92a promotes vascular smooth muscle cell proliferation and migration through the ROCK/MLCK signalling pathway.
Wang J, Zhang C, Li C, Zhao D, Li S, Ma L, Cui Y, Wei X, Zhao Y, Gao Y. Wang J, et al. J Cell Mol Med. 2019 May;23(5):3696-3710. doi: 10.1111/jcmm.14274. Epub 2019 Mar 25. J Cell Mol Med. 2019. PMID: 30907506 Free PMC article. - miR-20a regulates expression of the iron exporter ferroportin in lung cancer.
Babu KR, Muckenthaler MU. Babu KR, et al. J Mol Med (Berl). 2016 Mar;94(3):347-59. doi: 10.1007/s00109-015-1362-3. Epub 2015 Nov 12. J Mol Med (Berl). 2016. PMID: 26560875 Free PMC article. - HPV 16 E7 inhibits OSCC cell proliferation, invasion, and metastasis by upregulating the expression of miR-20a.
Hu J, Ge W, Xu J. Hu J, et al. Tumour Biol. 2016 Jul;37(7):9433-40. doi: 10.1007/s13277-016-4817-4. Epub 2016 Jan 19. Tumour Biol. 2016. PMID: 26781875 - Involvement of inflammation and its related microRNAs in hepatocellular carcinoma.
Jin K, Li T, Sánchez-Duffhues G, Zhou F, Zhang L. Jin K, et al. Oncotarget. 2017 Mar 28;8(13):22145-22165. doi: 10.18632/oncotarget.13530. Oncotarget. 2017. PMID: 27888618 Free PMC article. Review.
References
- Filipowicz W., Bhattacharyya S.N., Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. - PubMed
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. - PubMed
- Lewis B.P., Shih I.H., Jones-Rhoades M.W., Bartel D.P., Burge C.B. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. - PubMed
- Lu J., Getz G., Miska E.A., Alvarez-Saavedra E., Lamb J., Peck D., Sweet-Cordero A., Ebert B.L., Mak R.H., Ferrando A.A., Downing J.R., Jacks T., Horvitz H.R., Golub T.R. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA106280/CA/NCI NIH HHS/United States
- R01 CA134568/CA/NCI NIH HHS/United States
- CA106280/CA/NCI NIH HHS/United States
- R01 DK077776/DK/NIDDK NIH HHS/United States
- CA134568/CA/NCI NIH HHS/United States
- CA102325/CA/NCI NIH HHS/United States
- DK077776/DK/NIDDK NIH HHS/United States
- R56 DK077776/DK/NIDDK NIH HHS/United States
- R01 CA102325/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous