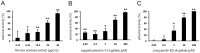3-O-galloylated procyanidins from Rumex acetosa L. inhibit the attachment of influenza A virus - PubMed (original) (raw)
3-O-galloylated procyanidins from Rumex acetosa L. inhibit the attachment of influenza A virus
Andrea Derksen et al. PLoS One. 2014.
Abstract
Infections by influenza A viruses (IAV) are a major health burden to mankind. The current antiviral arsenal against IAV is limited and novel drugs are urgently required. Medicinal plants are known as an abundant source for bioactive compounds, including antiviral agents. The aim of the present study was to characterize the anti-IAV potential of a proanthocyanidin-enriched extract derived from the aerial parts of Rumex acetosa (RA), and to identify active compounds of RA, their mode of action, and structural features conferring anti-IAV activity. In a modified MTT (MTTIAV) assay, RA was shown to inhibit growth of the IAV strain PR8 (H1N1) and a clinical isolate of IAV(H1N1)pdm09 with a half-maximal inhibitory concentration (IC50) of 2.5 µg/mL and 2.2 µg/mL, and a selectivity index (SI) (half-maximal cytotoxic concentration (CC50)/IC50)) of 32 and 36, respectively. At RA concentrations>1 µg/mL plaque formation of IAV(H1N1)pdm09 was abrogated. RA was also active against an oseltamivir-resistant isolate of IAV(H1N1)pdm09. TNF-α and EGF-induced signal transduction in A549 cells was not affected by RA. The dimeric proanthocyanidin epicatechin-3-O-gallate-(4β→8)-epicatechin-3'-O-gallate (procyanidin B2-di-gallate) was identified as the main active principle of RA (IC50 approx. 15 µM, SI≥13). RA and procyanidin B2-di-gallate blocked attachment of IAV and interfered with viral penetration at higher concentrations. Galloylation of the procyanidin core structure was shown to be a prerequisite for anti-IAV activity; o-trihydroxylation in the B-ring increased the anti-IAV activity. In silico docking studies indicated that procyanidin B2-di-gallate is able to interact with the receptor binding site of IAV(H1N1)pdm09 hemagglutinin (HA). In conclusion, the proanthocyanidin-enriched extract RA and its main active constituent procyanidin B2-di-gallate protect cells from IAV infection by inhibiting viral entry into the host cell. RA and procyanidin B2-di-gallate appear to be a promising expansion of the currently available anti-influenza agents.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. Structural features of flavan-3-ols, oligomeric proanthocyanidins, hydrolyzable tannins, depsides and building blocks of tannins tested for antiviral activity; compounds isolated from Rumex acetosa extract RA are marked by asterisk.
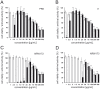
Figure 2. Antiviral and cytotoxic activity of RA on MDCK II cells.
1×104 pfu IAV/well in serum-free medium (antiviral activity, black bars) or serum-free medium (cytotoxic activity, white bars) were incubated with RA at different concentrations indicated for 1 h at 37°C. 48 h after adding the reaction mixtures to 96-well plates, the antiviral activity and cell vitality were determined by MTTIAV assay and cytotoxicity assay, respectively. The following IAV laboratory strains and isolates were used: (A) laboratory strain PR8 [A/Puerto Rico/8/34], (B) clinical isolate I1 [A(H1N1)pdm09], (C) clinical isolate NRW172 [A(H1N1)pdm09], (D) clinical isolate NRW173 [A(H1N1)pdm09]. Values represent mean ±SD of ≥3 independent experiments. * p<0.05, ** p<0.01 (two-tailed, unpaired Student's t-test). Statistical significance of antiviral activity was calculated for nontoxic concentrations only (A: 1 to 10 µg/mL, B: 1 to 7.5 µg/mL, C: 1 to 25 µg/mL, D: 1 to 10 µg/mL).
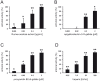
Figure 3. Reduction of IAV plaque formation by the Rumex acetosa extract RA (A), epigallocatechin-3-O-gallate (6) (B) and procyanidin B2-digallate (8) (C).
IAV and test compounds were co-incubated for 1 h at 37°C prior to the addition to MDCK II cells. Heparin served as positive control (D). Values (% of plaque reduction) ±SD relate to the respective mock-treated controls ( = 100%). * p<0.05, ** p<0.01 (two-tailed, unpaired Student's t-test).
Figure 4. Effect of Rumex acetosa extract RA (A), epigallocatechin-3-O-gallate (6) (B) and procyanidin B2-digallate (8) (C) on the penetration of IAV.
Effects on the penetration of IAV were determined by a modified plaque reduction assay. Test compounds were added for 30 min. after attachment of IAV to MDCK II cells at 4°C. Values (% of plaque reduction) ±SD relate to the respective mock-treated controls ( = 100%) and represent ≥3 independent experiments. * p<0.05, ** p<0.01 (two-tailed, unpaired Student's t-test).
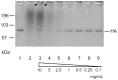
Figure 5. Effect of RA on the electrophoretic mobility of recombinant soluble HA.
Mock-treated HA (lane 1), RA (10 mg/mL) (lane 2), and HA treated with RA (0.1 to 10 mg/mL) as indicated for 1 h (lanes 3 to 9) were loaded onto 10% bis-tris SDS-PAGE gels and analyzed by Coomassie-staining. The positions of molecular weight marker (mwm) and HA are indicated. HA conglomerates in the gel pockets are marked by arrowhead.
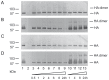
Figure 6. Effect of EGCG (6) (A, B) and procyanidin B2-di-gallate (8) (C, D) on electrophoretic mobility and detection of HA by immunoblotting.
Recombinant soluble HA was either mock-treated (lanes 1), incubated with EGCG (6) (2.18 mM) or procyanidin B2-di-gallate (8) (1.13 mM) dissolved in PBS for the times indicated (lanes 3 to 9) or incubated with PBS only (lanes 10 to 13); EGCG (6) (2.18 mM) and procyanidin B2-di-gallate (8) (1.13 mM) incubated in the absence of HA served as control (lanes 2). Figure 6A, C: Coomassie-stained SDS-PAGE. Figure 6B, D: Detection of HA by immunoblot using a penta-His-specific monoclonal antibody. The expected position of monomeric (approx. 75 kDa) and dimeric HA (approx. 150 kDa) is indicated. Required parameters are missing or incorrect.
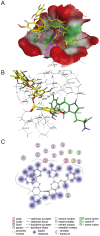
Figure 7. Protein-ligand docking of epicatechin-3-O-gallate-(4β→8)-epicatechin-3′-O-gallate (8) into the sialic acid binding cavity of hemagglutinin.
(A) 3D model; protein: green: hydrophobic, purple: polar, red: exposed; ligand: yellow: carbon, light grey: hydrogen, red: oxygen, blue: nitrogen; (B) Interactions of Tyr98 and Trp153; (C) 2D.
Figure 8. Influence of extract RA on TNF-α (A) and EGF (B) induced signal transduction in A549 cells.
Lanes 1 and 2 represent cells preincubated for 1 h with medium, lanes 3 and 4 with RA (100 µg/mL). (A) Stimulation of cells with TNF-α (20 ng/mL, 30 min.) (lane 2, 4), and detection of phosphorylated NF-κB; loading control β-actin; (B) stimulation of cells with EGF (30 ng/mL, 10 min.) (lane 2, 4), and detection of phosphorylated ERK1/2; loading control α-tubulin.
Similar articles
- Tackling the Future Pandemics: Broad-Spectrum Antiviral Agents (BSAAs) Based on A-Type Proanthocyanidins.
Maffei ME, Salata C, Gribaudo G. Maffei ME, et al. Molecules. 2022 Nov 30;27(23):8353. doi: 10.3390/molecules27238353. Molecules. 2022. PMID: 36500445 Free PMC article. Review. - Extract from Rumex acetosa L. for prophylaxis of periodontitis: inhibition of bacterial in vitro adhesion and of gingipains of Porphyromonas gingivalis by epicatechin-3-O-(4β→8)-epicatechin-3-O-gallate (procyanidin-B2-Di-gallate).
Schmuch J, Beckert S, Brandt S, Löhr G, Hermann F, Schmidt TJ, Beikler T, Hensel A. Schmuch J, et al. PLoS One. 2015 Mar 24;10(3):e0120130. doi: 10.1371/journal.pone.0120130. eCollection 2015. PLoS One. 2015. PMID: 25803708 Free PMC article. - Antiviral activity of hydroalcoholic extract from Eupatorium perfoliatum L. against the attachment of influenza A virus.
Derksen A, Kühn J, Hafezi W, Sendker J, Ehrhardt C, Ludwig S, Hensel A. Derksen A, et al. J Ethnopharmacol. 2016 Jul 21;188:144-52. doi: 10.1016/j.jep.2016.05.016. Epub 2016 May 10. J Ethnopharmacol. 2016. PMID: 27178637 - Oligomeric proanthocyanidins from Rumex acetosa L. inhibit the attachment of herpes simplex virus type-1.
Gescher K, Hensel A, Hafezi W, Derksen A, Kühn J. Gescher K, et al. Antiviral Res. 2011 Jan;89(1):9-18. doi: 10.1016/j.antiviral.2010.10.007. Epub 2010 Nov 9. Antiviral Res. 2011. PMID: 21070811 - Entry of influenza A virus into host cells - recent progress and remaining challenges.
Sempere Borau M, Stertz S. Sempere Borau M, et al. Curr Opin Virol. 2021 Jun;48:23-29. doi: 10.1016/j.coviro.2021.03.001. Epub 2021 Apr 7. Curr Opin Virol. 2021. PMID: 33838498 Review.
Cited by
- Tackling the Future Pandemics: Broad-Spectrum Antiviral Agents (BSAAs) Based on A-Type Proanthocyanidins.
Maffei ME, Salata C, Gribaudo G. Maffei ME, et al. Molecules. 2022 Nov 30;27(23):8353. doi: 10.3390/molecules27238353. Molecules. 2022. PMID: 36500445 Free PMC article. Review. - Phenol Derivatives Obtained from Grape Seed Extract Show Virucidal Activity against Murine Norovirus.
Kudkyal VR, Matsuura I, Hiramatsu H, Hayashi K, Kawahara T. Kudkyal VR, et al. Molecules. 2022 Nov 10;27(22):7739. doi: 10.3390/molecules27227739. Molecules. 2022. PMID: 36431850 Free PMC article. - Novel and Alternative Therapeutic Strategies for Controlling Avian Viral Infectious Diseases: Focus on Infectious Bronchitis and Avian Influenza.
Abbas G, Yu J, Li G. Abbas G, et al. Front Vet Sci. 2022 Jul 22;9:933274. doi: 10.3389/fvets.2022.933274. eCollection 2022. Front Vet Sci. 2022. PMID: 35937298 Free PMC article. Review. - Hypericum perforatum and Its Ingredients Hypericin and Pseudohypericin Demonstrate an Antiviral Activity against SARS-CoV-2.
Mohamed FF, Anhlan D, Schöfbänker M, Schreiber A, Classen N, Hensel A, Hempel G, Scholz W, Kühn J, Hrincius ER, Ludwig S. Mohamed FF, et al. Pharmaceuticals (Basel). 2022 Apr 25;15(5):530. doi: 10.3390/ph15050530. Pharmaceuticals (Basel). 2022. PMID: 35631357 Free PMC article. - Antiviral and Immunomodulatory Effects of Pelargonium sidoides DC. Root Extract EPs® 7630 in SARS-CoV-2-Infected Human Lung Cells.
Papies J, Emanuel J, Heinemann N, Kulić Ž, Schroeder S, Tenner B, Lehner MD, Seifert G, Müller MA. Papies J, et al. Front Pharmacol. 2021 Oct 25;12:757666. doi: 10.3389/fphar.2021.757666. eCollection 2021. Front Pharmacol. 2021. PMID: 34759825 Free PMC article.
References
- Lambert LC, Fauci AS (2010) Influenza vaccines for the future. N Engl J Med 363: 2036–2044. - PubMed
- Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, et al. (2011) Antiviral agents for the treatment and chemoprophylaxis of influenza --- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 60: 1–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
The authors acknowledge support by Deutsche Forschungsgemeinschaft and Open Access Publication Fund of University of Muenster. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources