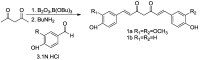Synthesis and biological evaluation of novel curcuminoid derivatives - PubMed (original) (raw)
Synthesis and biological evaluation of novel curcuminoid derivatives
Ya-Kun Cao et al. Molecules. 2014.
Abstract
Curcuminoids have been reported to possess multiple bioactivities, such as antioxidant, anticancer and anti-inflammatory properties. Three novel series of curcuminoid derivatives (11a-h, 15a-h and 19a-d) with enhanced bioactivity have been synthesized. Among the synthesized compounds, 11b exhibited the most significant activity with an MIC of 0.5 µM /mL against selected medically important Gram-positive cocci (S. aureus and S. viridans) and Gram-negative bacilli (E. coli and E. cloacae). The derivatives exhibited remarkable results in an antioxidant test with an IC50 2.4- to 9.3-folder smaller than curcuminoids. With respect to antiproliferative activity against Hep-G2, LX-2, SMMC7221 and MDA-MB-231, the derivatives exhibited an effect stronger than curcuminoids with an IC50 ranging from 0.18 to 4.25 µM.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
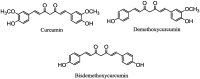
Figure 1
Curcumin, demethoxycurcumin and bisdemethoxycurcumin.
Scheme 1
Syntheses of curcumin (1a) and bisdemethoxycurcumin (1b).
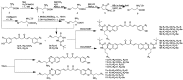
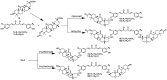
Scheme 2
Synthesis of selenomethionine-substituted curcuminoids and methionine-substituted curcuminoids.
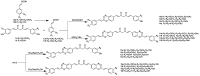
Scheme 3
Synthesis of curcuminoid-caffeic acid and curcuminoid-ferulic acid conjugates.
Scheme 4
Synthesis of curcuminoid-glycyrrhetinic acid conjugates.
Similar articles
- Synthesis and Biological Evaluation of Curcuminoid Derivatives.
Feng L, Li Y, Song ZF, Li HJ, Huai QY. Feng L, et al. Chem Pharm Bull (Tokyo). 2015;63(11):873-81. doi: 10.1248/cpb.c15-00470. Chem Pharm Bull (Tokyo). 2015. PMID: 26521852 - Biological evaluation and molecular docking studies of new curcuminoid derivatives: Synthesis and characterization.
Banuppriya G, Sribalan R, Padmini V, Shanmugaiah V. Banuppriya G, et al. Bioorg Med Chem Lett. 2016 Apr 1;26(7):1655-9. doi: 10.1016/j.bmcl.2016.02.066. Epub 2016 Feb 23. Bioorg Med Chem Lett. 2016. PMID: 26944612 - Biological activity, design, synthesis and structure activity relationship of some novel derivatives of curcumin containing sulfonamides.
Lal J, Gupta SK, Thavaselvam D, Agarwal DD. Lal J, et al. Eur J Med Chem. 2013 Jun;64:579-88. doi: 10.1016/j.ejmech.2013.03.012. Epub 2013 Mar 22. Eur J Med Chem. 2013. PMID: 23685942 - Synthesis of Curcuminoids and Evaluation of Their Cytotoxic and Antioxidant Properties.
Lozada-García MC, Enríquez RG, Ramírez-Apán TO, Nieto-Camacho A, Palacios-Espinosa JF, Custodio-Galván Z, Soria-Arteche O, Pérez-Villanueva J. Lozada-García MC, et al. Molecules. 2017 Apr 14;22(4):633. doi: 10.3390/molecules22040633. Molecules. 2017. PMID: 28420097 Free PMC article. - Synthesis of new uracil derivatives and their sugar hydrazones with potent antimicrobial, antioxidant and anticancer activities.
Nassar IF, Farargy AFE, Abdelrazek FM, Hamza Z. Nassar IF, et al. Nucleosides Nucleotides Nucleic Acids. 2020;39(7):991-1010. doi: 10.1080/15257770.2020.1736300. Epub 2020 Mar 4. Nucleosides Nucleotides Nucleic Acids. 2020. PMID: 32126887
Cited by
- A Review of Recent Curcumin Analogues and Their Antioxidant, Anti-Inflammatory, and Anticancer Activities.
Kaur K, Al-Khazaleh AK, Bhuyan DJ, Li F, Li CG. Kaur K, et al. Antioxidants (Basel). 2024 Sep 6;13(9):1092. doi: 10.3390/antiox13091092. Antioxidants (Basel). 2024. PMID: 39334750 Free PMC article. Review. - Application of Amino Acids in the Structural Modification of Natural Products: A Review.
Xu Q, Deng H, Li X, Quan ZS. Xu Q, et al. Front Chem. 2021 Apr 29;9:650569. doi: 10.3389/fchem.2021.650569. eCollection 2021. Front Chem. 2021. PMID: 33996749 Free PMC article. Review. - Formulation of the novel structure curcumin derivative-loaded solid lipid nanoparticles: synthesis, optimization, characterization and anti-tumor activity screening in vitro.
Li K, Pi C, Wen J, He Y, Yuan J, Shen H, Zhao W, Zeng M, Song X, Lee RJ, Wei Y, Zhao L. Li K, et al. Drug Deliv. 2022 Dec;29(1):2044-2057. doi: 10.1080/10717544.2022.2092235. Drug Deliv. 2022. PMID: 35775475 Free PMC article. - Synthesis and Anticancer Activities of Glycyrrhetinic Acid Derivatives.
Li Y, Feng L, Song ZF, Li HB, Huai QY. Li Y, et al. Molecules. 2016 Feb 6;21(2):199. doi: 10.3390/molecules21020199. Molecules. 2016. PMID: 26861280 Free PMC article. - Curcuminoid Chalcones: Synthesis and Biological Activity against the Human Colon Carcinoma (Caco-2) Cell Line.
Olender D, Sowa-Kasprzak K, Pawełczyk A, Skóra B, Zaprutko L, Szychowski KA. Olender D, et al. Curr Med Chem. 2024;31(33):5397-5416. doi: 10.2174/0109298673257972230919055832. Curr Med Chem. 2024. PMID: 37779412
References
- Civjan N. Chemical Biology: Approaches to Drug Discovery and Development to Targeting Disease. John Wiley & Sons; Hoboken, NJ, USA: 2012.
- Butler M.S. Natural products to drugs: Natural product derived compounds in clinical trials. Nat. Prod. Rep. 2005;22:162–195. - PubMed
- Chattopadhyay I., Biswas K., Bandyopadhyay U., Banerjee R.K. Turmeric and curcumin: Biological actions and medicinal applications. Curr. Sci. 2004;87:44–53.
- Motterlini R., Foresti R., Bassi R., Green C.J. Curcumin, an antioxidant and anti-inflammatory agent, induces heine oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic. Biol. Med. 2000;28:1303–1312. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous