MicroRNA-9 promotes tumor metastasis via repressing E-cadherin in esophageal squamous cell carcinoma - PubMed (original) (raw)
. 2014 Nov 30;5(22):11669-80.
doi: 10.18632/oncotarget.2581.
Jiangchao Li 2, Yinghui Zhu 1, Yongdong Dai 1, Tingting Zeng 1, Lulu Liu 1, Jianbiao Li 1, Hongbo Wang 1, Yanru Qin 3, Musheng Zeng 1, Xin-Yuan Guan 4, Yan Li 1
Affiliations
- PMID: 25375090
- PMCID: PMC4294333
- DOI: 10.18632/oncotarget.2581
MicroRNA-9 promotes tumor metastasis via repressing E-cadherin in esophageal squamous cell carcinoma
Ye Song et al. Oncotarget. 2014.
Abstract
MicroRNAs (miRNAs) play a critical role in development and progression of cancers. Deregulation of MicroRNA-9 (miR-9) has been documented in many types of cancers but their role in the development of esophageal squamous cell carcinoma (ESCC) has not been studied. This study aimed to investigate the effect of miR-9 in esophageal cancer metastasis. The up-regulation of miR-9 was frequently detected in primary ESCC tumor tissue, which was significantly associated with clinical progression (P = 0.022), lymph node metastasis (P = 0.007) and poor overall survival (P < 0.001). Functional study demonstrated that miR-9 promoted cell migration and tumor metastasis, which were effectively inhibited when expression of miR-9 was silenced. Moreover, we demonstrated that miR-9 interacted with the 3'-untranslated region of E-cadherin and down-regulated its expression, which induced β-catenin nuclear translocation and subsequently up-regulated c-myc and CD44 expression. In addition, miR-9 induced epithelial-mesenchymal transition (EMT) in ESCC, a key event in tumor metastasis. Taken together, our study demonstrates that miR-9 plays an important role in ESCC metastasis by activating β-catenin pathway and inducing EMT via targeting E-cadherin. Our study also suggests miR-9 can be served as a new independent prognostic marker and/or as a novel potential therapeutic target for ESCC.
Conflict of interest statement
Conflicts of interests
The authors declared that no conflict of interest exists.
Figures
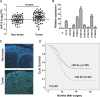
Figure 1. miR-9 was frequently up-regulated in primary ESCC cases and cell lines
(A) qRT-PCR shows that miR-9 was frequently up-regulated in 67 primary ESCC tissues compared with their adjacent non-tumor tissues. (P = 0.0016, independent t test). Expression of miR-9 was shown in log10 scale and normalized to U6. (B) Up-regulation of miR-9 was detected in all tested ESCC cell lines except EC109 compared with pool of non-tumor tissues (N). U6 was set as an endogenous control. *P < 0.05; **P < 0.001. (C) Representative, of miR-9 expression (green signals) in a pair of ESCC tumor tissue and corresponding non-tumor tissue detected by MISH. Nuclei were conterstained by DAPI (blue color). Original magnification, 40 × objective. (D) Kaplan-Meier analysis indicates up-regulation of miR-9 was significantly associated with poorer overall survival rates of ESCC patients (P < 0.001).
Figure 2. Functional study of miR-9
(A) Relative expression of miR-9 was detected by qRT-PCR in _miR-9_-transfected (R9) HKESC1 and KYSE410 cells compared with empty vector-transfected cells (V). **P < 0.001. (B) Foci formation assay was performed to compare frequency of foci formation between _miR-9_- and empty vector-transfected cells. Results are expressed as mean ±S.E.M. of three independent experiments. *P < 0.05. (C) Representative images of xenografts and summary of tumor weight in tumor formation in nude mice. _miR-9_- and empty vector-transfected cells were inoculated subcutaneously to the flanks of nude mice. Xenografts were isolated and weighted after 4 weeks.
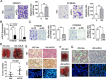
Figure 3. miR-9 promotes cell migration and tumor metastasis
(A) Representative images and summary of cell migration assay. Compared with empty vector-transfected cells, _miR-9_-transfected cells could promote cell migration in HKESC1 and KYSE410 cells. The results are expressed as mean ± S.E.M. of three independent experiments. **P < 0.01. (B) Down-regulation of miR-9 was detected by qRT-PCR when miR-9 was silenced by a siRNA in KYSE30 and KYSE510 cells. Cells treated with scramble siRNA were used as control cells. Results are expressed as mean ± S.E.M. of three independent experiments. **P < 0.01. (C) Representative images and summary of migrated cells between siRNA-miR-9 and scramble siRNA treated KYSE30 and KYSE510 cells. The results are expressed as mean ± S.E.M. of three independent experiments. **P < 0.01. (D) miR-9 could promote ESCC metastasis in vivo. Representative images of lungs derived from mice injected with HK-miR-9 and control cells (upper). Visible tumor nodules were counted and summarized (bottom). (E) H&E staining (upper) and IHC staining of CK (middle) was performed on pulmonary sections derived from mice. Original magnification: 20 × objective. Expression of miR-9 (green signals) was detected by MISH in pulmonary sections (bottom). Original magnification: 40 × objective. (F) Representative image of lung derived from mice injected with 410-miR-9 and control cells. (G) H&E staining (upper) was performed on pulmonary sections derived from mice injected with 410-miR-9 and control cells. Original magnification: 40 × objective. Expression of miR-9 was detected by MISH in pulmonary sections (bottom). Original magnification: 20 × objective.
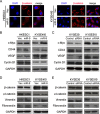
Figure 4. miR-9 down-regulates E-cadherin in ESCC cells
(A) qRT-PCR and Western blotting results showed that miR-9 could effectively down-regulate E-cadherin expression in HK-miR-9 and 410-miR-9 cells compared with control cells. qRT-PCR results were expressed as mean ± S.E.M. of three independent experiments. **P < 0.001. GAPDH was set as an internal control in Western blot analysis. (B) Expressions of E-cadherin in both mRNA and protein levels were increased when miR-9 was silenced in KYSE30 and KYSE510 cells by a siRNA against miR-9, compared with scramble siRNA-treated cells. C, control scramble siRNA; si, siRNA against miR-9. **P < 0.01. (C) Luciferase assay was performed to confirm that miR-9 could target E-cadherin. miR-9 overexpressed cells and control cells were co-transfected with pMIR-REPORT-CDH1(3′-UTR)/empty vector with pRL-TK, and relative luciferase activity was detected. *P < 0.05. (D) Representative pictures of E-cadherin staining (by IHC) and miR-9 (by MISH) in a pair of ESCC tumor tissue and corresponding non-tumor tissue. Results showed that E-cadherin was down-regulated in miR-9 overexpressed tumor tissue. Original magnification: 20 × objective.
Figure 5. miR-9 activates β-catenin pathway and induces EMT
(A) IF showed that nuclear translocation of β-catenin (red) happened in miR-9-transfected cells while β-catenin located mainly on the membrane in empty vector-transfected cells. Nuclei were counterstained with DAPI. Original magnification: 40 × objective. (B-E) Western blot analysis was used to compare protein expression levels between miR-9- and empty vector-transfected cells (B and D), or between cells treated with siRNA against miR-9 and scramble siRNA (C and E). GAPDH was set as internal control.
Similar articles
- Downregulation of MiR-31 stimulates expression of LATS2 via the hippo pathway and promotes epithelial-mesenchymal transition in esophageal squamous cell carcinoma.
Gao Y, Yi J, Zhang K, Bai F, Feng B, Wang R, Chu X, Chen L, Song H. Gao Y, et al. J Exp Clin Cancer Res. 2017 Nov 16;36(1):161. doi: 10.1186/s13046-017-0622-1. J Exp Clin Cancer Res. 2017. PMID: 29145896 Free PMC article. - Overexpression of miR-191 Predicts Poor Prognosis and Promotes Proliferation and Invasion in Esophageal Squamous Cell Carcinoma.
Gao X, Xie Z, Wang Z, Cheng K, Liang K, Song Z. Gao X, et al. Yonsei Med J. 2017 Nov;58(6):1101-1110. doi: 10.3349/ymj.2017.58.6.1101. Yonsei Med J. 2017. PMID: 29047233 Free PMC article. - MiR-424-5p participates in esophageal squamous cell carcinoma invasion and metastasis via SMAD7 pathway mediated EMT.
Wang F, Wang J, Yang X, Chen D, Wang L. Wang F, et al. Diagn Pathol. 2016 Sep 15;11(1):88. doi: 10.1186/s13000-016-0536-9. Diagn Pathol. 2016. PMID: 27628042 Free PMC article. - P21, COX-2, and E-cadherin are potential prognostic factors for esophageal squamous cell carcinoma.
Lin Y, Shen LY, Fu H, Dong B, Yang HL, Yan WP, Kang XZ, Dai L, Zhou HT, Yang YB, Liang Z, Chen KN. Lin Y, et al. Dis Esophagus. 2017 Feb 1;30(2):1-10. doi: 10.1111/dote.12522. Dis Esophagus. 2017. PMID: 27868288 Review. - CD44 expression is predictive of poor prognosis in pharyngolaryngeal cancer: systematic review and meta-analysis.
Chai L, Liu H, Zhang Z, Wang F, Wang Q, Zhou S, Wang S. Chai L, et al. Tohoku J Exp Med. 2014 Jan;232(1):9-19. doi: 10.1620/tjem.232.9. Tohoku J Exp Med. 2014. PMID: 24429392 Review.
Cited by
- miR-9 promotes cell proliferation and inhibits apoptosis by targeting LASS2 in bladder cancer.
Wang H, Zhang W, Zuo Y, Ding M, Ke C, Yan R, Zhan H, Liu J, Wang J. Wang H, et al. Tumour Biol. 2015 Dec;36(12):9631-40. doi: 10.1007/s13277-015-3713-7. Epub 2015 Jul 7. Tumour Biol. 2015. PMID: 26150338 - miR-9-5p Exerts a Dual Role in Cervical Cancer and Targets Transcription Factor TWIST1.
Babion I, Jaspers A, van Splunter AP, van der Hoorn IAE, Wilting SM, Steenbergen RDM. Babion I, et al. Cells. 2019 Dec 26;9(1):65. doi: 10.3390/cells9010065. Cells. 2019. PMID: 31888045 Free PMC article. - MicroRNAs: A novel signature in the metastasis of esophageal squamous cell carcinoma.
Wei QY, Jin F, Wang ZY, Li BJ, Cao WB, Sun ZY, Mo SJ. Wei QY, et al. World J Gastroenterol. 2024 Mar 21;30(11):1497-1523. doi: 10.3748/wjg.v30.i11.1497. World J Gastroenterol. 2024. PMID: 38617454 Free PMC article. Review. - Roles of microRNAs in tumorigenesis and metastasis of esophageal squamous cell carcinoma.
Cui D, Cheung AL. Cui D, et al. World J Clin Oncol. 2021 Aug 24;12(8):609-622. doi: 10.5306/wjco.v12.i8.609. World J Clin Oncol. 2021. PMID: 34513596 Free PMC article. Review. - Noncoding RNA Expression Aberration Is Associated with Cancer Progression and Is a Potential Biomarker in Esophageal Squamous Cell Carcinoma.
Sugihara H, Ishimoto T, Miyake K, Izumi D, Baba Y, Yoshida N, Watanabe M, Baba H. Sugihara H, et al. Int J Mol Sci. 2015 Nov 24;16(11):27824-34. doi: 10.3390/ijms161126060. Int J Mol Sci. 2015. PMID: 26610479 Free PMC article. Review.
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: a cancer journal for clinicians. 2005;55:74–108. - PubMed
- Yang CS. Research on esophageal cancer in China: a review. Cancer Res. 1980;40:2633–2644. - PubMed
- Enzinger PC, Mayer RJ. Esophageal cancer. The New England journal of medicine. 2003;349:2241–2252. - PubMed
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA: a cancer journal for clinicians. 2007;57:43–66. - PubMed
- He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nature reviews Genetics. 2004;5:522–531. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous