Human neural stem cells rapidly ameliorate symptomatic inflammation in early-stage ischemic-reperfusion cerebral injury - PubMed (original) (raw)
- PMID: 25418536
- PMCID: PMC4445985
- DOI: 10.1186/scrt519
Human neural stem cells rapidly ameliorate symptomatic inflammation in early-stage ischemic-reperfusion cerebral injury
Lei Huang et al. Stem Cell Res Ther. 2014.
Abstract
Introduction: Clinically, a good deal of injury from stroke results from ischemic-reperfusion. There is a loss of cerebral parenchyma and its associated cells, disruption of neuronal connections, compromise of the blood-brain barrier, and inflammation. We tested whether exogenously engrafted human neural stem cells could migrate rapidly and extensively to damaged regions, following transplantation into a neurogenic site where migration cues are already underway during stroke onset, then counteract a number of these pathological processes.
Methods: One day post-injury, we injected human neural stem cells (hNSCs) into the ipsilesional hippocampus of a mouse model of stroke with middle cerebral artery occlusion to induce focal ischemia followed by reperfusion (MCAO/R). The time frame for hNSC transplantation corresponded to upregulation of endogenous proinflammatory cytokines. We examined the effect of hNSC transplantation on pathological processes and behavioral dysfunction 48 hours post-injury.
Results: Twenty-four hours after transplantation, engrafted hNSCs had migrated extensively to the lesion, and infarct volume was reduced relative to MCAO/R controls. The behavioral deficits seen in MCAO/R controls were also significantly improved. Given this rapid response, we hypothesized that the mechanisms underlying therapeutic activity were anti-inflammatory rather than due to cell replacement. In support of this idea, in hNSC-transplanted mice we observed reduced microglial activation, decreased expression of proinflammatory factors (tumor necrosis factor-α, interleukin (IL)-6, IL-1β, monocyte chemotactic protein-1, macrophage inflammatory protein-1α) and adhesion molecules (intercellular adhesion molecule-1, vascular cell adhesion molecule-1), and amelioration of blood-brain barrier damage.
Conclusions: While long-term effects of engrafted hNSCs on the amelioration of ischemic stroke-induced behavioral dysfunction in a rodent model have been reported, our study is the first to show rapid, beneficial impacts on behavioral function (within 24 hours) upon early delivery of hNSCs into the hippocampus.
Figures
Figure 1
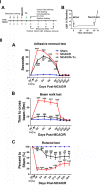
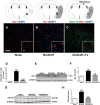
Human NSC transplantation ameliorates behavioral deficits. I. (A) Experimental timeline. MCAO/R was performed in C57BL/6 J mice at time 0, and hNSCs were transplanted into the ipsilesional hippocampus 24 hours later. Outcomes were assessed at the indicated intervals. Tx, transplantation. (B) Relative cerebral blood flow (rCBF) was measured by laser Doppler flowmetry over the area supplied by the MCA. Pre-ischemic rCBF was assigned a value of 100%. Subsequent values are presented as a percentage of that value. MCAO promoted an 80% decrease in rCBF (86.1 ± 1.6%), and the reperfusion flow rate 60 minutes post-MCAO was 90.1 ± 4.0% (n = 18). II. (A) In the forepaw adhesive removal tests, the mean adhesive removal time for hNSC-transplanted mice was significantly shorter than that of MCAO/R mice, showing improvement (n = 14). (B) In the beam walk tests, hNSC-transplanted MCAO/R mice improved neurological dysfunction as shown by crossing the beam in significantly less time than did nontransplanted MCAO/R mice (n = 14). (C) In the rotarod tests, hNSC-transplanted mice improved motor dysfunction as shown by remaining on the rod for a significantly longer time than did nontransplanted MCAO/R mice (n = 14). Four velocities (10, 15, 20, and 25 rpm) were used, and three trials were performed at each velocity. Durations at all speeds are summed, and data are presented as a percentage relative to the sham mice. **P <0.01, ***P <0.001 versus sham mice; ## P <0.01; ### P <0.001 versus nontransplanted MCAO/R mice. Data are expressed as mean ± SEM. MCA, middle cerebral artery; MCAO/R, middle cerebral artery occlusion with reperfusion; NSC, neural stem cells; SEM, standard error of the mean.
Figure 2
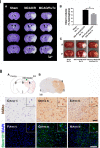
Human NSCs engraft widely and reduce infarct volume. I. (A) Infarct volume was reduced in hNSC-transplanted mice (MCAO/R + Tx) 24 hours post-transplantation (neurons are stained with cresyl violet, purple; infarct, white). (B) Quantification of II A. (n = 4, *P <0.05). (C) TTC staining (white, infarct). Shown are two representative samples of the same brain from anterior to posterior. A, anterior; P, posterior. II. (A) Diagram of brain and injection site. Arrowhead indicates NSC injection site; red dots, hNSC-disseminated areas. Ipsi, ipsilesional; Contra, contralesional. (B-H) hNSCs were identified with two different human-specific antibodies (human mitochondria, hMito or human cytoplasmic protein, Stem121). Three sampling sites are shown in rectangles, (C-H) higher magnification images: area 1, (no infarct), area 2 (infarct center), area 3 (peri-infarct). (B-E) Immunoreactivity to human mitochondria (hMito; DAB, brown) and (F-H) to Stem121 (G, H: green). Scale bars: B, 1 mm; E, 50 μm (10 μm inset); H, 100 μm (10 μm inset). DAB, 3,3-diaminobenzidine; MCAO/R, middle cerebral artery occlusion with reperfusion; NSCs, neural stem cells; TTC, triphenyl tetrazolium chloride; Tx: Transplantation.
Figure 3
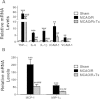
Human NSC transplantation reduces proinflammatory gene expression. (A) Reduced inflammatory marker expression in the ipsilesional hemisphere of hNSC-transplanted MCAO/R brains. Expression of inflammatory genes was measured by RT-PCR and normalized to that of GAPDH. Transcript levels of proinflammatory cytokines TNF-α, IL-6, IL-1β, and cell adhesion molecules, ICAM-1 and VCAM-1, are elevated in MCAO/R brains and downregulated in MCAO/R + hNSC-transplanted brains compared with MCAO/R brains. **P <0.01, ***P <0.001 versus sham mice; ## P <0.01, ### P <0.001 versus MCAO/R mice. (B) Chemokines MCP-1 and MIP-1α are dramatically elevated in the ipsilesional hemisphere 48 hours post-MCAO/R and significantly downregulated in hNSC-transplanted brains. **P <0.01, ***P <0.001 versus sham mice; ## P <0.01, ### P <0.001 versus MCAO/R mice. All data are expressed as mean ± SEM (n = 5). MCAO/R, middle cerebral artery occlusion with reperfusion; NSC, neural stem cells; SEM, standard error of the mean; Tx, transplantation.
Figure 4
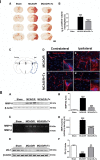
Human NSC transplantation ameliorates BBB leakage. I. (A) Representative images of IgG staining in sham, MCAO/R and MCAO/R + hNSC-transplanted (Tx) mice showing BBB damage. Scale bar, 2 mm. (B) Quantification of IgG staining, expressed as a percentage of the volume showing IgG-positive staining per ipsilesional hemisphere (n = 5, ***P <0.001 versus sham; # P <0.05 versus MCAO/R). (C) Brain diagram. (D) The spatial distribution of intravenously administered Texas Red dextran 70 kDa (red) in contralateral (a, c) and ipsilateral (b, d) cortical regions of stroke mice with/without hNSC transplantation 48 hours post-MCAO/R. Reduced extravasation was observed in hNSC-transplanted mice. Scale bar, 10 μm (10 μm, inset). II. (A) Western blot analysis showing MMP-9 protein level in the ipsilesional cortex. (B) Quantification of A (n = 6, ***P <0.001 versus sham; ### _P <_0.001 versus MCAO/R). (C) Zymography assay showing MMP-9 activity in the ipsilesional cortex. (D) Quantification of C (n = 6, ***P <0.001 versus sham; ### P <0.001 versus MCAO/R). (E) Western blot analysis of ZO-1. (F) Quantification of E (n = 4, ***P <0.001 versus sham; ## P <0.01 versus MCAO/R). BBB, blood-brain barrier; IgG, immunoglobulin G; MCAO/R, middle cerebral artery occlusion with reperfusion; MMP-9, matrix metalloproteinase-9; NSC, neural stem cells; ZO-1, zona occluden.
Figure 5
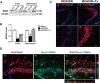
Human NSC transplantation reduces microglial activation. Immunofluorescence staining for Iba-1 (red), a microglial marker, and Stem121, an hNSC marker, (A) sham, (B) MCAO/R, and (C) MCAO/R + hNSC engrafted mice (Insets, higher magnification). Microglial activation was increased 48 hours post-MCAO/R, while hNSC transplantation suppressed activation by 24 hours post-transplantation (arrowhead, hNSC transplantation site). (D) Quantification of Iba-1-positive active microglia per area (0.15 mm2) in different mouse groups (n = 4, *P <0.05, ***P <0.001 versus sham; ### P <0.001 versus MCAO/R). (E) Western blot analysis showing the Iba-1 protein level in the ipsilesional cortex. (F) Quantification of E (n = 3, ***, P <0.001 versus sham group; ##, P <0.01 versus MCAO/R group. (G) Western blot analysis showing the CD11b protein level in the ipsilesional cortex. (H) Quantification of G (n = 3, ****, P <0.0001 versus sham group; ####, P <0.0001 versus MCAO/R group). Data expressed as mean ± SEM. Rectangles indicate sampling regions. Gray region indicates infarct. MCAO/R, middle cerebral artery occlusion with reperfusion; NSC, neural stem cell; SEM, standard error of the mean.
Figure 6
Human NSC transplantation increases BNDF expression in the ipsilesional hemisphere. (A) Representative Western blot showing BDNF expression in the cortex and hippocampus. (B) Quantification of A. (n = 4; **P <0.01, ***P <0.001 versus sham; ## P <0.01, ### P <0.001 versus MCAO/R). (C) Representative confocal photomicrographs showing BDNF (red) immunofluorescence in the ipsilesional hippocampus. DAPI staining is shown in blue (c, d: High magnification of rectangles shown in a, b, respectively). Scale bar, 100 μm (a, b); 30 μm (c, d). (D) BDNF-positive endogenous cells (red), Stem121-positive hNSCs (green), and BDNF-positive hNSCs, as indicated by arrow (arrow, yellow; merge). Scale bar, 30 μm. BNDF, brain-derived neurotrophic factor; DAPI, 4′,6-diamidino-2-phenylindole; MCAO/R, middle cerebral artery occlusion with reperfusion; NSC, neural stem cell.
Similar articles
- Human neural stem cells improve early stage stroke outcome in delayed tissue plasminogen activator-treated aged stroke brains.
Boese AC, Eckert A, Hamblin MH, Lee JP. Boese AC, et al. Exp Neurol. 2020 Jul;329:113275. doi: 10.1016/j.expneurol.2020.113275. Epub 2020 Mar 5. Exp Neurol. 2020. PMID: 32147438 Free PMC article. - Modulation of gene expression on a transcriptome-wide level following human neural stem cell transplantation in aged mouse stroke brains.
Hamblin MH, Murad R, Yin J, Vallim G, Lee JP. Hamblin MH, et al. Exp Neurol. 2022 Jan;347:113913. doi: 10.1016/j.expneurol.2021.113913. Epub 2021 Nov 6. Exp Neurol. 2022. PMID: 34752785 Free PMC article. - Bystander Effect Fuels Human Induced Pluripotent Stem Cell-Derived Neural Stem Cells to Quickly Attenuate Early Stage Neurological Deficits After Stroke.
Eckert A, Huang L, Gonzalez R, Kim HS, Hamblin MH, Lee JP. Eckert A, et al. Stem Cells Transl Med. 2015 Jul;4(7):841-51. doi: 10.5966/sctm.2014-0184. Epub 2015 May 29. Stem Cells Transl Med. 2015. PMID: 26025980 Free PMC article. - The effects of hyperbaric oxygen therapy on the brain with middle cerebral artery occlusion.
Thiankhaw K, Chattipakorn N, Chattipakorn SC. Thiankhaw K, et al. J Cell Physiol. 2021 Mar;236(3):1677-1694. doi: 10.1002/jcp.29955. Epub 2020 Jul 21. J Cell Physiol. 2021. PMID: 32692455 Review. - The role of inflammation after acute stroke: utility of pursuing anti-adhesion molecule therapy.
DeGraba TJ. DeGraba TJ. Neurology. 1998 Sep;51(3 Suppl 3):S62-8. doi: 10.1212/wnl.51.3_suppl_3.s62. Neurology. 1998. PMID: 9744839 Review.
Cited by
- Harnessing the anti-inflammatory properties of stem cells for transplant therapy in hemorrhagic stroke.
Corey S, Bonsack B, Heyck M, Shear A, Sadanandan N, Zhang H, Borlongan CV. Corey S, et al. Brain Hemorrhages. 2020 Mar;1(1):24-33. doi: 10.1016/j.hest.2019.12.005. Epub 2020 Jan 22. Brain Hemorrhages. 2020. PMID: 34056567 Free PMC article. - Signaling pathways involved in ischemic stroke: molecular mechanisms and therapeutic interventions.
Qin C, Yang S, Chu YH, Zhang H, Pang XW, Chen L, Zhou LQ, Chen M, Tian DS, Wang W. Qin C, et al. Signal Transduct Target Ther. 2022 Jul 6;7(1):215. doi: 10.1038/s41392-022-01064-1. Signal Transduct Target Ther. 2022. PMID: 35794095 Free PMC article. Review. - Human neural stem cells improve early stage stroke outcome in delayed tissue plasminogen activator-treated aged stroke brains.
Boese AC, Eckert A, Hamblin MH, Lee JP. Boese AC, et al. Exp Neurol. 2020 Jul;329:113275. doi: 10.1016/j.expneurol.2020.113275. Epub 2020 Mar 5. Exp Neurol. 2020. PMID: 32147438 Free PMC article. - Stem cells technology: a powerful tool behind new brain treatments.
Duru LN, Quan Z, Qazi TJ, Qing H. Duru LN, et al. Drug Deliv Transl Res. 2018 Oct;8(5):1564-1591. doi: 10.1007/s13346-018-0548-y. Drug Deliv Transl Res. 2018. PMID: 29916013 Review. - The Effects and Underlying Mechanisms of Cell Therapy on Blood-Brain Barrier Integrity After Ischemic Stroke.
Gao L, Song Z, Mi J, Hou P, Xie C, Shi J, Li Y, Manaenko A. Gao L, et al. Curr Neuropharmacol. 2020;18(12):1213-1226. doi: 10.2174/1570159X18666200914162013. Curr Neuropharmacol. 2020. PMID: 32928089 Free PMC article.
References
- Lee JP, Jeyakumar M, Gonzalez R, Takahashi H, Lee PJ, Baek RC, Clark D, Rose H, Fu G, Clarke J, McKercher S, Meerloo J, Muller FJ, Park KI, Butters TD, Dwek RA, Schwartz P, Tong G, Wenger D, Lipton SA, Seyfried TN, Platt FM, Snyder EY. Stem cells act through multiple mechanisms to benefit mice with neurodegenerative metabolic disease. Nat Med. 2007;13:439–447. doi: 10.1038/nm1548. - DOI - PubMed
- Jeyakumar M, Lee JP, Sibson NR, Lowe JP, Stuckey DJ, Tester K, Fu G, Newlin R, Smith DA, Snyder EY, Platt FM. Neural stem cell transplantation benefits a monogenic neurometabolic disorder during the symptomatic phase of disease. Stem Cells. 2009;27:2362–2370. doi: 10.1002/stem.163. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials