Methods to analyze treatment effects in the presence of missing data for a continuous heavy drinking outcome measure when participants drop out from treatment in alcohol clinical trials - PubMed (original) (raw)
Randomized Controlled Trial
. 2014 Nov;38(11):2826-34.
doi: 10.1111/acer.12543.
Collaborators, Affiliations
- PMID: 25421518
- PMCID: PMC4244651
- DOI: 10.1111/acer.12543
Randomized Controlled Trial
Methods to analyze treatment effects in the presence of missing data for a continuous heavy drinking outcome measure when participants drop out from treatment in alcohol clinical trials
Katie Witkiewitz et al. Alcohol Clin Exp Res. 2014 Nov.
Abstract
Background: Attrition is common in alcohol clinical trials and the resultant loss of data represents an important methodological problem. In the absence of a simulation study, the drinking outcomes among those who are lost to follow-up are not known. Individuals who drop out of treatment and continue to provide drinking data, however, may be a reasonable proxy group for making inferences about the drinking outcomes of those lost to follow-up.
Methods: We used data from the COMBINE study, a multisite, randomized clinical trial, to examine drinking during the 4 months of treatment among individuals who dropped out of treatment but continued to provide drinking data (i.e., "treatment dropouts;" n = 185). First, we estimated the observed treatment effect size for naltrexone versus placebo in a sample that included both treatment completers (n = 961) and treatment dropouts (n = 185; total N = 1,146), as well as the observed treatment effect size among just those who dropped out of treatment (n = 185). In both the total sample (N = 1,146) and the dropout sample (n = 185), we then deleted the drinking data after treatment dropout from those 185 individuals to simulate missing data. Using the deleted data sets, we then estimated the effect of naltrexone on the continuous outcome percent heavy drinking days using 6 methods to handle missing data (last observation carried forward, baseline observation carried forward, placebo mean imputation, missing = heavy drinking days, multiple imputation (MI), and full information maximum likelihood [FIML]).
Results: MI and FIML produced effect size estimates that were most similar to the true effects observed in the full data set in all analyses, while missing = heavy drinking days performed the worst.
Conclusions: Although missing drinking data should be avoided whenever possible, MI and FIML yield the best estimates of the treatment effect for a continuous outcome measure of heavy drinking when there is dropout in an alcohol clinical trial.
Keywords: Alcohol Use Disorder; Clinical Trials; Continuous Outcome Measure; Missing data; Relapse; Treatment.
Copyright © 2014 by the Research Society on Alcoholism.
Figures
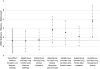
Figure 1
Estimates from the mixed model of percent heavy drinking days in treatment completers and treatment dropouts (“Actual Effect” among Completers and Dropouts – Complete; n = 1146; solid line) and for the data from the treatment dropouts where post dropout drinking data was set to missing (“Deleted Group” Completers and Dropouts – Deleted; dashed-dotted line) using missing data methods (standard error bars based on the data in Table 3). The dashed horizontal line represents “true” (i.e., “actual effect”) placebo-naltrexone difference based on the Treatment Completers and Dropouts – Complete sample.
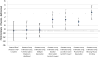
Figure 2
Estimates from mixed model of percent heavy drinking days among treatment dropouts (“Actual Effect” among Treatment Dropouts Only – Complete; n = 185; solid line) and for the data from the treatment dropouts where post dropout drinking data was set to missing (“Deleted Group” Treatment Dropouts Only – Deleted; dashed-dotted line) using missing data methods (standard error bars based on the data in Table 4). The dashed horizontal line represents “true” (i.e., “actual effect”) placebo-naltrexone difference based on the Treatment Completers and Dropouts – Complete sample.
Similar articles
- Missing Data in Alcohol Clinical Trials with Binary Outcomes.
Hallgren KA, Witkiewitz K, Kranzler HR, Falk DE, Litten RZ, O'Malley SS, Anton RF; In conjunction with the Alcohol Clinical Trials Initiative (ACTIVE) Workgroup. Hallgren KA, et al. Alcohol Clin Exp Res. 2016 Jul;40(7):1548-57. doi: 10.1111/acer.13106. Epub 2016 Jun 2. Alcohol Clin Exp Res. 2016. PMID: 27254113 Free PMC article. - Missing data in alcohol clinical trials: a comparison of methods.
Hallgren KA, Witkiewitz K. Hallgren KA, et al. Alcohol Clin Exp Res. 2013 Dec;37(12):2152-60. doi: 10.1111/acer.12205. Epub 2013 Jul 24. Alcohol Clin Exp Res. 2013. PMID: 23889334 Free PMC article. - Effectiveness and treatment moderators of internet interventions for adult problem drinking: An individual patient data meta-analysis of 19 randomised controlled trials.
Riper H, Hoogendoorn A, Cuijpers P, Karyotaki E, Boumparis N, Mira A, Andersson G, Berman AH, Bertholet N, Bischof G, Blankers M, Boon B, Boß L, Brendryen H, Cunningham J, Ebert D, Hansen A, Hester R, Khadjesari Z, Kramer J, Murray E, Postel M, Schulz D, Sinadinovic K, Suffoletto B, Sundström C, de Vries H, Wallace P, Wiers RW, Smit JH. Riper H, et al. PLoS Med. 2018 Dec 18;15(12):e1002714. doi: 10.1371/journal.pmed.1002714. eCollection 2018 Dec. PLoS Med. 2018. PMID: 30562347 Free PMC article. - The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking.
Pettinati HM, O'Brien CP, Rabinowitz AR, Wortman SP, Oslin DW, Kampman KM, Dackis CA. Pettinati HM, et al. J Clin Psychopharmacol. 2006 Dec;26(6):610-25. doi: 10.1097/01.jcp.0000245566.52401.20. J Clin Psychopharmacol. 2006. PMID: 17110818 Review. - Short of complete abstinence: an analysis exploration of multiple drinking episodes in alcoholism treatment trials.
Wang SJ, Winchell CJ, McCormick CG, Nevius SE, O'Neill RT. Wang SJ, et al. Alcohol Clin Exp Res. 2002 Dec;26(12):1803-9. doi: 10.1097/01.ALC.0000042009.07691.12. Alcohol Clin Exp Res. 2002. PMID: 12500103 Review.
Cited by
- Efficacy of Extended-Release Naltrexone on HIV-Related and Drinking Outcomes Among HIV-Positive Patients: A Randomized-Controlled Trial.
Edelman EJ, Moore BA, Holt SR, Hansen N, Kyriakides TC, Virata M, Brown ST, Justice AC, Bryant KJ, Fiellin DA, Fiellin LE. Edelman EJ, et al. AIDS Behav. 2019 Jan;23(1):211-221. doi: 10.1007/s10461-018-2241-z. AIDS Behav. 2019. PMID: 30073637 Free PMC article. Clinical Trial. - Missing Data in Alcohol Clinical Trials with Binary Outcomes.
Hallgren KA, Witkiewitz K, Kranzler HR, Falk DE, Litten RZ, O'Malley SS, Anton RF; In conjunction with the Alcohol Clinical Trials Initiative (ACTIVE) Workgroup. Hallgren KA, et al. Alcohol Clin Exp Res. 2016 Jul;40(7):1548-57. doi: 10.1111/acer.13106. Epub 2016 Jun 2. Alcohol Clin Exp Res. 2016. PMID: 27254113 Free PMC article. - ACTing towards better living during COVID-19: The effects of Acceptance and Commitment therapy for individuals affected by COVID-19.
Shepherd K, Golijani-Moghaddam N, Dawson DL. Shepherd K, et al. J Contextual Behav Sci. 2022 Jan;23:98-108. doi: 10.1016/j.jcbs.2021.12.003. Epub 2021 Dec 16. J Contextual Behav Sci. 2022. PMID: 34931160 Free PMC article. - Predicting implementation of the PAX Good Behavior Game + MyTeachingPartner interventions.
Braun SS, Bradshaw CP, Beahm LA, Budavari AC, Downer J, Ialongo NS, Tolan PH. Braun SS, et al. Front Psychol. 2023 Mar 10;14:1059138. doi: 10.3389/fpsyg.2023.1059138. eCollection 2023. Front Psychol. 2023. PMID: 36968753 Free PMC article. - How Much Is Too Much? Patterns of Drinking During Alcohol Treatment and Associations With Post-Treatment Outcomes Across Three Alcohol Clinical Trials.
Witkiewitz K, Roos CR, Pearson MR, Hallgren KA, Maisto SA, Kirouac M, Forcehimes AA, Wilson AD, Robinson CS, McCallion E, Tonigan JS, Heather N. Witkiewitz K, et al. J Stud Alcohol Drugs. 2017 Jan;78(1):59-69. doi: 10.15288/jsad.2017.78.59. J Stud Alcohol Drugs. 2017. PMID: 27936365 Free PMC article.
References
- Anton RF, Randall CL. Measurement and choice of drinking outcome variables in the COMBINE Study. Journal of Studies on Alcohol - Supplement. 2005;15:104–109. - PubMed
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. - PubMed
- Barnes SA, Larsen MD, Schroeder D, Hanson A, Decker PA. Missing data assumptions and methods in a smoking cessation study. Addiction. 2010;105:431–437. - PubMed
- COMBINE Study Research Group. Testing combined pharmacotherapies and behavioral interventions in alcohol dependence: rationale and methods. Alcohol Clin Exp Res. 2003;27:1107–1122. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical