Granulocyte colony-stimulating factor attenuates delayed tPA-induced hemorrhagic transformation in ischemic stroke rats by enhancing angiogenesis and vasculogenesis - PubMed (original) (raw)
Granulocyte colony-stimulating factor attenuates delayed tPA-induced hemorrhagic transformation in ischemic stroke rats by enhancing angiogenesis and vasculogenesis
Ike C dela Peña et al. J Cereb Blood Flow Metab. 2015 Feb.
Abstract
Treatment with tissue plasminogen activator (tPA) beyond the therapeutic time window (>4.5 hours post stroke) may produce hemorrhagic transformation (HT). Strategies that could extend the narrow time window of tPA will benefit a significant number of stroke patients. Male Sprague-Dawley rats underwent middle cerebral artery occlusion (MCAo) and given vehicle, tPA (10 mg/kg), or tPA and granulocyte colony-stimulating factor (G-CSF, 300 μg/kg), at 6 hours after MCAo. Twenty-four hours post treatment, G-CSF+tPA-treated stroke rats displayed 25% improvement in neurological functions and 38.9% reduction of hemorrhage, with Western blots showing 1.9- and 1.2-fold increments in Ang-2 expression in the ischemic cortex and striatum, respectively, and 3-fold increase in phosphorylated endothelial nitric oxide synthase expression in the ipsilateral cortex relative to tPA-treated rats. Immunohistochemistry also showed 2- and 2.8-fold increase in von-Willebrand expression, 3.2- and 2.2-fold increased CD34+ expression, and 4- and 13-fold upregulation of VEGFR-2 expression in the ischemic cortex and striatum, respectively, in G-CSF+tPA-treated stroke rats relative to tPA-treated subjects. Altogether, these findings indicate that G-CSF attenuated delayed tPA-induced HT likely via the enhancement of angiogenesis and vasculogenesis. The use of G-CSF to protect the vasculature may improve the clinical outcome of tPA even outside the currently indicated therapeutic window for ischemic stroke.
Figures
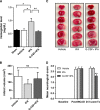
Figure 1
Effects of granulocyte colony-stimulating factor (G-CSF) on delayed tissue plasminogen activator (tPA)-induced hemorrhage, cerebral infarction, and neurological deficits in MCAo rats. (A) Quantitative analysis of cerebral hemorrhage volume with spectrophotometric assay revealed incidence of hemorrhage (shown as increased levels of hemoglobin levels in the brain) in rats subjected to delayed tPA treatment. G-CSF treatment caused a 38% reduction of delayed tPA-induced hemorrhage (_n_=5–6 animals per group). (B) Quantitative analysis of infarct volume in vehicle- (_n_=9), tPA- (_n_=7) and G-CSF+tPA- (_n_=8) treated groups. (C) Photographs are representative coronal brain sections stained with triphenyltetrazolium chloride 24 hours after MCAo, showing infarct area (white) and intact areas (red). G-CSF had no effect on infarct volume. (D) Effects of G-CSF on delayed tPA-induced neurological deficits in MCAo rats. Twenty-four hours after drug treatment (Tx), rats injected with G-CSF displayed improvement of delayed tPA-induced neurological deficits (25% and 24.8%, relative to control and tPA-treated stroke rats, respectively). (_n_=8–10 animals per group), *P<0.05, **P<0.01; NS, not significant. Data are expressed as mean±s.e.m.
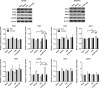
Figure 2
Western blotting for angiopoetins (Ang-1 and Ang-2), endothelial nitric oxide synthase (eNOS) and phosphorylated-eNOS. As shown in representative bands, treatment with granulocyte colony-stimulating factor+tissue plasminogen activator (G-CSF+tPA) upregulated Ang-2 (marker of angiogenesis) expression in the ischemic hemisphere. Quantitative analyses showed 1.9- and 1.2-fold increment in Ang-2 expression in the ischemic cortex and striatum, respectively, in G-CSF+tPA-treated rats relative to subjects treated with tPA only. Ang-1 expression in both ipsilateral and contralateral hemispheres was similar in all groups. Relative to tPA-treated rats, G-CSF+tPA-treated stroke rats also showed threefold increase in peNOS expression in the ischemic cortex, but not striatum, in cerebral hemisphere ipsilateral to MCAo. Total eNOS expression in the ipsilateral and contralateral hemispheres was similar in all treatment groups although there was a trend of increased eNOS expression in the cortex of G-CSF+tPA-treated versus tPA-treated rats. *P<0.05, **P<0.01, ***P<0.001 _n_=5–6. Data are expressed as mean±s.e.m. relative to sham group and normalized to _β_-actin.
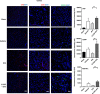
Figure 3
Immunohistochemical analyses of von-Willebrand (vWF), CD34+ and vascular endothelial growth factor (VEGRF)-2 expression levels in the ischemic cortex. Representative merged images showing co-localization of vWF, CD34+, or VEGFR-2 with 4′,6-diamidino-2-phenylindole (DAPI; blue filter, nuclear staining). Analyses of fluorescence intensities showed 2-fold increase in the expression of vascular marker vWF, as well as 3.2- and 4-fold increment in endothelial progenitor cell markers CD34+ and VEGFR-2, respectively, in the ischemic cortex of G-CSF+tPA-treated rats compared with subjects administered with tPA only (at 6 hours post MCAO). **P<0.01, _n_=5–6. Data are expressed as mean±s.e.m. Horizontal bar indicates 100 _μ_M.
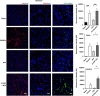
Figure 4
Immunohistochemical analyses of von-Willebrand (vWF), CD34+ and vascular endothelial growth factor (VEGRF)-2 expression levels in the ischemic striatum. Representative merged images showing co-localization of vWF, CD34+, or VEGFR-2 with 4′,6-diamidino-2-phenylindole (DAPI; blue filter, nuclear staining). Analysis of fluorescence intensities showed 2.8-fold increase in the expression of vascular marker vWF, as well as 2.2- and 13-fold increment in endothelial progenitor cell markers CD34+ and VEGFR-2, respectively, in the ischemic cortex of G-CSF+tPA-treated rats compared with subjects administered with tPA only (at 6 hours post MCAo). *P<0.05, **P<0.01, _n_=5–6. Data are expressed as mean±s.e.m. Horizontal bar indicates 100 _μ_M.
Similar articles
- Extension of Tissue Plasminogen Activator Treatment Window by Granulocyte-Colony Stimulating Factor in a Thromboembolic Rat Model of Stroke.
Dela Peña IC, Yang S, Shen G, Fang Liang H, Solak S, Borlongan CV. Dela Peña IC, et al. Int J Mol Sci. 2018 May 31;19(6):1635. doi: 10.3390/ijms19061635. Int J Mol Sci. 2018. PMID: 29857523 Free PMC article. - GSK-3β inhibitor TWS119 attenuates rtPA-induced hemorrhagic transformation and activates the Wnt/β-catenin signaling pathway after acute ischemic stroke in rats.
Wang W, Li M, Wang Y, Li Q, Deng G, Wan J, Yang Q, Chen Q, Wang J. Wang W, et al. Mol Neurobiol. 2016 Dec;53(10):7028-7036. doi: 10.1007/s12035-015-9607-2. Epub 2015 Dec 15. Mol Neurobiol. 2016. PMID: 26671619 Free PMC article. - Baicalin Attenuates Blood-Brain Barrier Disruption and Hemorrhagic Transformation and Improves Neurological Outcome in Ischemic Stroke Rats with Delayed t-PA Treatment: Involvement of ONOO--MMP-9 Pathway.
Chen H, Guan B, Chen X, Chen X, Li C, Qiu J, Yang D, Liu KJ, Qi S, Shen J. Chen H, et al. Transl Stroke Res. 2018 Oct;9(5):515-529. doi: 10.1007/s12975-017-0598-3. Epub 2017 Dec 23. Transl Stroke Res. 2018. PMID: 29275501 - Adjunctive Therapy Approaches for Ischemic Stroke: Innovations to Expand Time Window of Treatment.
Knecht T, Story J, Liu J, Davis W, Borlongan CV, Dela Peña IC. Knecht T, et al. Int J Mol Sci. 2017 Dec 19;18(12):2756. doi: 10.3390/ijms18122756. Int J Mol Sci. 2017. PMID: 29257093 Free PMC article. Review. - Therapeutic Strategies to Attenuate Hemorrhagic Transformation After Tissue Plasminogen Activator Treatment for Acute Ischemic Stroke.
Kanazawa M, Takahashi T, Nishizawa M, Shimohata T. Kanazawa M, et al. J Atheroscler Thromb. 2017 Mar 1;24(3):240-253. doi: 10.5551/jat.RV16006. Epub 2016 Dec 13. J Atheroscler Thromb. 2017. PMID: 27980241 Free PMC article. Review.
Cited by
- Dynamics of Endothelial Cell Diversity and Plasticity in Health and Disease.
Larionov A, Hammer CM, Fiedler K, Filgueira L. Larionov A, et al. Cells. 2024 Jul 29;13(15):1276. doi: 10.3390/cells13151276. Cells. 2024. PMID: 39120307 Free PMC article. Review. - A brief physical activity protects against ischemic stroke.
Zhang H, Lee JY, Borlongan CV, Tajiri N. Zhang H, et al. Brain Circ. 2019 Sep 30;5(3):112-118. doi: 10.4103/bc.bc_32_19. eCollection 2019 Jul-Sep. Brain Circ. 2019. PMID: 31620657 Free PMC article. Review. - Granulocyte-Colony Stimulating Factor (G-CSF) for stroke: an individual patient data meta-analysis.
England TJ, Sprigg N, Alasheev AM, Belkin AA, Kumar A, Prasad K, Bath PM. England TJ, et al. Sci Rep. 2016 Nov 15;6:36567. doi: 10.1038/srep36567. Sci Rep. 2016. PMID: 27845349 Free PMC article. Review. - Oxidative stress and DNA damage after cerebral ischemia: Potential therapeutic targets to repair the genome and improve stroke recovery.
Li P, Stetler RA, Leak RK, Shi Y, Li Y, Yu W, Bennett MVL, Chen J. Li P, et al. Neuropharmacology. 2018 May 15;134(Pt B):208-217. doi: 10.1016/j.neuropharm.2017.11.011. Epub 2017 Nov 8. Neuropharmacology. 2018. PMID: 29128308 Free PMC article. Review. - Colony-Stimulating Factors on Mobilizing CD34+ Cells and Improving Neurological Functions in Patients With Stroke: A Meta-Analysis and a Systematic Review.
Chen X, Sun W, Zhong P, Wu D. Chen X, et al. Front Pharmacol. 2021 Jul 22;12:704509. doi: 10.3389/fphar.2021.704509. eCollection 2021. Front Pharmacol. 2021. PMID: 34366857 Free PMC article. Review.
References
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nature Rev Neurosci. 2003;4:399–415. - PubMed
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A. Thrombolysis with alteplase 3 to 4.5 h after acute ischemic stroke. N Eng J Med. 2008;359:1317–1329. - PubMed
- The NINDS rt-PA Stroke Study Group Intracerebral hemorrhage after intravenous tPA therapy for ischemic stroke. Stroke. 1997;2007;28:2109–2118. - PubMed
- Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35:2726–2730. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS071956/NS/NINDS NIH HHS/United States
- R21 NS089851/NS/NINDS NIH HHS/United States
- 1R01NS071956-01A1/NS/NINDS NIH HHS/United States
- 1R21NS089851/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous