A role for partial endothelial-mesenchymal transitions in angiogenesis? - PubMed (original) (raw)
Review
A role for partial endothelial-mesenchymal transitions in angiogenesis?
Katrina M Welch-Reardon et al. Arterioscler Thromb Vasc Biol. 2015 Feb.
Abstract
The contribution of epithelial-to-mesenchymal transitions (EMT) in both developmental and pathological conditions has been widely recognized and studied. In a parallel process, governed by a similar set of signaling and transcription factors, endothelial-to-mesenchymal transitions (EndoMT) contribute to heart valve formation and the generation of cancer-associated fibroblasts. During angiogenic sprouting, endothelial cells express many of the same genes and break down basement membrane; however, they retain intercellular junctions and migrate as a connected train of cells rather than as individual cells. This has been termed a partial endothelial-to-mesenchymal transition. A key regulatory check-point determines whether cells undergo a full or a partial epithelial-to-mesenchymal transitions/endothelial-to-mesenchymal transition; however, very little is known about how this switch is controlled. Here we discuss these developmental/pathological pathways, with a particular focus on their role in vascular biology.
Keywords: EMT; angiogenesis; endothelial; transcription factor.
© 2014 American Heart Association, Inc.
Conflict of interest statement
Disclosure
The authors declare no conflicts of interest
Figures
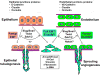
Figure 1
Complete vs. partial EMT/EndoMT. Epithelial and endothelial cells comprise the quiescent epithelium and endothelium respectively and utilize junctional proteins to maintain connections. Once transcriptional reprogramming is initiated, an event led by the EMT/EndoMT-transcription factors Slug, Snail, Twist and Zeb1/2, the epithelial/endothelial cells lose apical-basal polarity, sever intercellular junctions and become motile cells. However, the regulatory signal(s) that determine whether these cells undergo a complete EMT/EndoMT or partial EMT/EndoMT remains unclear. In the case of sprouting angiogenesis, the contact-dependent Notch signaling pathway may have a major role to play in this process.
Similar articles
- Soluble factors regulated by epithelial-mesenchymal transition mediate tumour angiogenesis and myeloid cell recruitment.
Suarez-Carmona M, Bourcy M, Lesage J, Leroi N, Syne L, Blacher S, Hubert P, Erpicum C, Foidart JM, Delvenne P, Birembaut P, Noël A, Polette M, Gilles C. Suarez-Carmona M, et al. J Pathol. 2015 Aug;236(4):491-504. doi: 10.1002/path.4546. Epub 2015 May 12. J Pathol. 2015. PMID: 25880038 - Endothelial-Mesenchymal Transition in Cardiovascular Disease.
Alvandi Z, Bischoff J. Alvandi Z, et al. Arterioscler Thromb Vasc Biol. 2021 Sep;41(9):2357-2369. doi: 10.1161/ATVBAHA.121.313788. Epub 2021 Jul 1. Arterioscler Thromb Vasc Biol. 2021. PMID: 34196216 Free PMC article. Review. - Nerve growth factor as an angiogenic factor.
Nico B, Mangieri D, Benagiano V, Crivellato E, Ribatti D. Nico B, et al. Microvasc Res. 2008 Mar;75(2):135-41. doi: 10.1016/j.mvr.2007.07.004. Epub 2007 Jul 21. Microvasc Res. 2008. PMID: 17764704 Review. - The morphological and molecular mechanisms of epithelial/endothelial-to-mesenchymal transition and its involvement in atherosclerosis.
Wesseling M, Sakkers TR, de Jager SCA, Pasterkamp G, Goumans MJ. Wesseling M, et al. Vascul Pharmacol. 2018 Jul;106:1-8. doi: 10.1016/j.vph.2018.02.006. Epub 2018 Feb 20. Vascul Pharmacol. 2018. PMID: 29471141 Review. - Myofibroblasts in proliferative diabetic retinopathy can originate from infiltrating fibrocytes and through endothelial-to-mesenchymal transition (EndoMT).
Abu El-Asrar AM, De Hertogh G, van den Eynde K, Alam K, Van Raemdonck K, Opdenakker G, Van Damme J, Geboes K, Struyf S. Abu El-Asrar AM, et al. Exp Eye Res. 2015 Mar;132:179-89. doi: 10.1016/j.exer.2015.01.023. Epub 2015 Jan 28. Exp Eye Res. 2015. PMID: 25637870
Cited by
- "How to Release or Not Release, That Is the Question." A Review of Interleukin-1 Cellular Release Mechanisms in Vascular Inflammation.
Kidder E, Gangopadhyay S, Francis S, Alfaidi M. Kidder E, et al. J Am Heart Assoc. 2024 Mar 5;13(5):e032987. doi: 10.1161/JAHA.123.032987. Epub 2024 Feb 23. J Am Heart Assoc. 2024. PMID: 38390810 Free PMC article. Review. - The therapeutic potential of targeting the endothelial-to-mesenchymal transition.
Man S, Sanchez Duffhues G, Ten Dijke P, Baker D. Man S, et al. Angiogenesis. 2019 Feb;22(1):3-13. doi: 10.1007/s10456-018-9639-0. Epub 2018 Aug 3. Angiogenesis. 2019. PMID: 30076548 Free PMC article. Review. - An In Vitro Platform to Study Reversible Endothelial-to-Mesenchymal Transition.
Krishnamoorthi MK, Thandavarayan RA, Youker KA, Bhimaraj A. Krishnamoorthi MK, et al. Front Pharmacol. 2022 Jun 23;13:912660. doi: 10.3389/fphar.2022.912660. eCollection 2022. Front Pharmacol. 2022. PMID: 35814231 Free PMC article. - EGR1 Regulation of Vasculogenic Mimicry in the MDA-MB-231 Triple-Negative Breast Cancer Cell Line through the Upregulation of KLF4 Expression.
Jung E, Lee YH, Ou S, Kim TY, Shin SY. Jung E, et al. Int J Mol Sci. 2023 Sep 21;24(18):14375. doi: 10.3390/ijms241814375. Int J Mol Sci. 2023. PMID: 37762678 Free PMC article. - Quantitative proteomic profiling of tumor-associated vascular endothelial cells in colorectal cancer.
Wang G, Yang Q, Li M, Zhang Y, Cai Y, Liang X, Fu Y, Xiao Z, Zhou M, Xie Z, Huang H, Huang Y, Chen Y, He Q, Peng F, Chen Z. Wang G, et al. Biol Open. 2019 May 13;8(5):bio042838. doi: 10.1242/bio.042838. Biol Open. 2019. PMID: 31036754 Free PMC article.
References
- Thiery JP, Acloque H, Huang RY, Nieto MAs. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. - PubMed