MALAT1 promotes colorectal cancer cell proliferation/migration/invasion via PRKA kinase anchor protein 9 - PubMed (original) (raw)
MALAT1 promotes colorectal cancer cell proliferation/migration/invasion via PRKA kinase anchor protein 9
Min-Hui Yang et al. Biochim Biophys Acta. 2015 Jan.
Abstract
Our previous studies have shown that the 3' end of metastasis associated lung adenocarcinoma transcript 1 (MALAT1) is involved in colorectal cancer (CRC) cell proliferation and migration/invasion in vitro. The role and mechanism of MALAT1 in CRC metastasis in vivo, however, remain largely unknown. In the present study, we found that MALAT1 was up-regulated in human primary CRC tissues with lymph node metastasis. Overexpression of MALAT1 via RNA activation promoted CRC cell proliferation, invasion and migration in vitro, and stimulated tumor growth and metastasis in mice in vivo. Conversely, knockdown of MALAT1 inhibited CRC tumor growth and metastasis. MALAT1 regulated at least 243 genes in CRC cells in a genome-wide expression profiling. Among these genes, PRKA kinase anchor protein 9 (AKAP-9) was significantly up-regulated at both mRNA and protein levels. AKAP-9 was highly expressed in CRC cells with metastatic potential and human primary CRC tissues with lymph node metastasis, but not in normal cells or tissues. Importantly, knockdown of AKAP-9 blocked MALAT1-mediated CRC cell proliferation, migration and invasion. These data indicate that MALAT1 may promote CRC tumor development via its target protein AKAP-9.
Keywords: Colorectal cancer; Long non-coding RNA; Metastasis; Metastasis associated lung adenocarcinoma transcript 1; PRKA kinase anchor protein 9.
Copyright © 2014 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of Interest Statement: None declared.
Figures
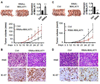
Figure 1. MALAT1 was up-regulated in human CRC tissues with metastasis
(A) Semi-quantitative analyses of MALAT1 levels in human CRC tissues. MALAT1 expression was higher in CRCs (T) than adjacent normal tissues (N). The highest level of MALAT1 was observed in CRCs with lymph metastasis (L) in all groups. (B) MALAT1 expression in human CRC tissues was detected by real-time PCR and normalized to GAPDH. MALAT1 levels in all 9 groups (CRC, CRC with lymph metastasis (LymCRC), and adjacent normal tissues) were consistent with the semi-quantitative analyses shown in A. *P<0.05 compared to normal tissue group; **P<0.05 compared to CRC and normal tissue group. (C) MALAT1 expression in human CRCs with and without metastasis relative to match-normal tissues. The level of MALAT1 in human CRCs with metastasis (mCRC, n=9) was significantly higher than that in CRCs without metastasis (nmCRC, n=18).
Figure 2. RNA activation-mediated overexpression of MALAT1 promoted CRC cell proliferation and invasion
(A) Schematic locations of small activation RNA (saRNA) target sites relative to the transcription starting site of MALAT1 gene including CpG island, Ctrl (scramble control), P1 (saRNA targeted in MALAT1 promoter region −592 bp), P2 (saRNA targeted in MALAT1 promoter region −348 bp), M1 (saRNA targeted in MALAT1 mRNA +258 bp), M2 (saRNA targeted in MALAT1 mRNA +380 bp), M3 (saRNA targeted in MALAT1 mRNA +1474 bp), M4 (saRNA targeted in MALAT1 mRNA +2584 bp). (B–C) saRNA-induced MALAT1 expression. SW480 cells were transfected with scramble (Ctrl) or different saRNAs (P1, P2, m1–m4). MALAT1 expression were assessed by semi-quantitative analysis (B) and real-time PCR (C). GAPDH was used as an internal control. (D, E) SW620 and SW480 cells expressed an elevated level of MALAT1 due to RNA activation (RNAa) as detected by semi-quantitative (D) and real-time PCR (E). (F) RNAa-activated MALAT1 promoted CRC cell proliferation as measured by CCK-8 assay. (G) RNAa-activated MALAT1 promoted CRC cell invasion as determined by matrigel invasion chamber analysis. *P < 0.05, **P <0.01 compared to corresponding control cells (Ctrl) (n=3).
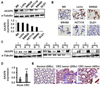
Figure 3. MALAT1 promoted CRC tumor growth in vivo
SW480 cells with scramble RNA (Ctrl), stable overexpression of MALAT1 (RNAa-MALAT), or MALAT1 knockdown (RNAi-MALAT1) were injected subcutaneously into nude mice. Tumors were allowed to grow for 30 days. (A) External whole-body images, MALAT1 expression in 30 day-old tumors, and quantification of the tumor sizes. Tumors derived from RNAa-MALAT1 cells grew significantly faster than that from control cells. *P<0.05 compared to the control groups in each time point. **P<0.01 compared to the control group at 30 day after injection (n=6). (B) Representative H&E images and immunohistochemical Ki-67 staining of tumor tissues derived from control (Ctrl) or MALAT1- overexpressed cells (RNAa-MALAT1). (C) Tumor of the RNAi-MALAT1 cells grew much slower than that of control cells. *P<0.05 compared to MALAT1 knockdown group (RNAi-MALAT1). **P<0.01 compared to the control group at 30 day after injection (n=6). (D) Representative H&E images and immunohistochemical Ki-67 staining of tumor tissues derived from control (Ctrl) or MALAT1-blocked (RNAi-MALAT1) cells. RNAa-mediated MALAT1 overexpression stimulated while MALAT1 knockdown inhibited CRC cell proliferation in vivo.
Figure 4. MALAT1 enhanced tumor invasion and metastasis in vivo
Small cubes of SW480 tumors grew from cells with stable overexpression of MALAT1 (RNAa-MALAT1), stable knockdown of MALAT1 (RNAi-MALAT1), or left untreated (Ctrl) were implanted into the caecum terminus of nude mice as described in the Methods. P denotes primary tumor. T and arrows indicate the metastatic nodules. (A) Tissue images of metastasis. RNAa-activated MALAT1 expression promoted while MALAT1 knockdown by RNAi inhibited CRC metastasis to lung and liver. (B) Histological images of metastatic nodules in organs. Xenograft tumors with MALAT1 overexpression significantly promoted the invasion of implanted tumor in colon and metastasis in liver and lung. (C) Incidence of metastasis in mice implanted with SW480 cells containing scramble RNA (Ctrl), RNAa-MALAT1, or RNAi-MALAT1 as indicated.
Figure 5. AKAP-9 expressed in CRC cells and tumor tissues
(A) AKAP-9 protein expression in six CRC cell lines was detected by western blot and normalized to α-Tubulin expression. (B) AKAP-9 expression in six CRC cell lines was examined by immunocytochemistry staining. (C) AKAP-9 expression was detected in nine human CRC tumor tissues. AKAP-9 levels in CRC tumor (T) was significantly higher than adjacent normal tissues (N) as detected by western blot. (D) Quantitative analysis of AKAP-9 expression shown in C by normalizing to α-Tubulin. **P<0.001 compared to normal tissue (Norm), n=9. (E) AKAP-9 expression in CRC tumor tissue was detected by immunohistochemistry staining.
Figure 6. AKAP-9 was required for MALAT1-mediated CRC cell growth, migration and invasion
SW480 cells were co-transfected control (RNAaCtrl) or MALAT1 saRNA (RNAa) with scramble (shCtrl) or AKAP-9 shRNA (shAKAP9) as indicated. Cell proliferation, migration and invasion were measured. (A) AKAP-9 expression was increased by RNAa activation but knocked down by shAKAP9. **P<0.01 (n=3). (B) Cell proliferation was detected by MTT assay. MALAT1 activation stimulated the SW480 cell proliferation, which was attenuated when AKAP-9 was knocked down. **P<0.01 compared to other two groups (n=3). (C) Cell migration was measured by wound-healing assay. Relative migration distance was 24 normalized to the original gap produced by inserts (0 h). **P<0.01 (n=3). (D) SW480 cell invasion was determined by matrigel invasion assay. MALAT1 induction enhanced SW480 cell invasion, which was attenuated by silencing of AKAP9. **P<0.01 (n=3).
Similar articles
- Long non-coding RNA MALAT1 increases AKAP-9 expression by promoting SRPK1-catalyzed SRSF1 phosphorylation in colorectal cancer cells.
Hu ZY, Wang XY, Guo WB, Xie LY, Huang YQ, Liu YP, Xiao LW, Li SN, Zhu HF, Li ZG, Kan H. Hu ZY, et al. Oncotarget. 2016 Mar 8;7(10):11733-43. doi: 10.18632/oncotarget.7367. Oncotarget. 2016. PMID: 26887056 Free PMC article. - AKAP-9 promotes colorectal cancer development by regulating Cdc42 interacting protein 4.
Hu ZY, Liu YP, Xie LY, Wang XY, Yang F, Chen SY, Li ZG. Hu ZY, et al. Biochim Biophys Acta. 2016 Jun;1862(6):1172-81. doi: 10.1016/j.bbadis.2016.03.012. Epub 2016 Apr 12. Biochim Biophys Acta. 2016. PMID: 27039663 Free PMC article. - Long non-coding RNA MALAT1 promotes tumour growth and metastasis in colorectal cancer through binding to SFPQ and releasing oncogene PTBP2 from SFPQ/PTBP2 complex.
Ji Q, Zhang L, Liu X, Zhou L, Wang W, Han Z, Sui H, Tang Y, Wang Y, Liu N, Ren J, Hou F, Li Q. Ji Q, et al. Br J Cancer. 2014 Aug 12;111(4):736-48. doi: 10.1038/bjc.2014.383. Epub 2014 Jul 15. Br J Cancer. 2014. PMID: 25025966 Free PMC article. - MALAT1: A Promising Therapeutic Target for the Treatment of Metastatic Colorectal Cancer.
Uthman YA, Ibrahim KG, Abubakar B, Bello MB, Malami I, Imam MU, Qusty N, Cruz-Martins N, Batiha GE, Abubakar MB. Uthman YA, et al. Biochem Pharmacol. 2021 Aug;190:114657. doi: 10.1016/j.bcp.2021.114657. Epub 2021 Jun 16. Biochem Pharmacol. 2021. PMID: 34144008 Review. - MALAT1 in colorectal cancer: Its implication as a diagnostic, prognostic, and predictive biomarker.
Cervena K, Vodenkova S, Vymetalkova V. Cervena K, et al. Gene. 2022 Nov 15;843:146791. doi: 10.1016/j.gene.2022.146791. Epub 2022 Aug 9. Gene. 2022. PMID: 35961438 Review.
Cited by
- Long noncoding RNAs in cancer: mechanisms of action and technological advancements.
Bartonicek N, Maag JL, Dinger ME. Bartonicek N, et al. Mol Cancer. 2016 May 27;15(1):43. doi: 10.1186/s12943-016-0530-6. Mol Cancer. 2016. PMID: 27233618 Free PMC article. Review. - MALAT1-miR-124-RBG2 axis is involved in growth and invasion of HR-HPV-positive cervical cancer cells.
Liu S, Song L, Zeng S, Zhang L. Liu S, et al. Tumour Biol. 2016 Jan;37(1):633-40. doi: 10.1007/s13277-015-3732-4. Epub 2015 Aug 5. Tumour Biol. 2016. PMID: 26242259 - Long Noncoding RNA MALAT1 Regulates Hepatocellular Carcinoma Growth Under Hypoxia via Sponging MicroRNA-200a.
Zhao ZB, Chen F, Bai XF. Zhao ZB, et al. Yonsei Med J. 2019 Aug;60(8):727-734. doi: 10.3349/ymj.2019.60.8.727. Yonsei Med J. 2019. PMID: 31347327 Free PMC article. - LOC646329 long non-coding RNA sponges miR-29b-1 and regulates TGFβ signaling in colorectal cancer.
Javanmard AR, Dokanehiifard S, Bohlooli M, Soltani BM. Javanmard AR, et al. J Cancer Res Clin Oncol. 2020 May;146(5):1205-1215. doi: 10.1007/s00432-020-03145-6. Epub 2020 Feb 7. J Cancer Res Clin Oncol. 2020. PMID: 32034483 - lncRNA Malat1 and miR-26 cooperate in the regulation of neuronal progenitor cell proliferation and differentiation.
Was N, Sauer M, Fischer U, Becker M. Was N, et al. RNA. 2022 Oct 27;29(1):69-81. doi: 10.1261/rna.079436.122. Online ahead of print. RNA. 2022. PMID: 36302652 Free PMC article.
References
- Mercer TR, Mattick JS. Structure and function of long noncoding RNAs in epigenetic regulation. Nat Struct Mol Biol. 2013;20(3):300–307. - PubMed
- Nagano T, Fraser P. No-nonsense functions for long noncoding RNAs. Cell. 2011;145(2):178–181. - PubMed
- Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136(4):629–641. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical