A role for human mitochondrial complex II in the production of reactive oxygen species in human skin - PubMed (original) (raw)
A role for human mitochondrial complex II in the production of reactive oxygen species in human skin
Alasdair Anderson et al. Redox Biol. 2014.
Abstract
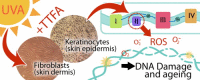
The mitochondrial respiratory chain is a major generator of cellular oxidative stress, thought to be an underlying cause of the carcinogenic and ageing process in many tissues including skin. Previous studies of the relative contributions of the respiratory chain (RC) complexes I, II and III towards production of reactive oxygen species (ROS) have focussed on rat tissues and certainly not on human skin which is surprising as this tissue is regularly exposed to UVA in sunlight, a potent generator of cellular oxidative stress. In a novel approach we have used an array of established specific metabolic inhibitors and DHR123 fluorescence to study the relative roles of the mitochondrial RC complexes in cellular ROS production in 2 types of human skin cells. These include additional enhancement of ROS production by exposure to physiological levels of UVA. The effects within epidermal and dermal derived skin cells are compared to other tissue cell types as well as those harbouring a compromised mitochondrial status (Rho-zero A549). The results show that the complex II inhibitor, TTFA, was the only RC inhibitor to significantly increase UVA-induced ROS production in both skin cell types (P<0.05) suggesting that the role of human skin complex II in terms of influencing ROS production is more important than previously thought particularly in comparison to liver cells. Interestingly, two-fold greater maximal activity of complex II enzyme was observed in both skin cell types compared to liver (P<0.001). The activities of RC enzymes appear to decrease with increasing age and telomere length is correlated with ageing. Our study showed that the level of maximal complex II activity was higher in the MRC5/hTERT (human lung fibroblasts transfected with telomerase) cells than the corresponding wild type cells (P=0.0012) which can be considered (in terms of telomerase activity) as models of younger and older cells respectively.
Keywords: Ageing; Mitochondria; Reactive oxygen species (ROS); Respiratory chain; Skin.
Copyright © 2014 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
Graphical abstract
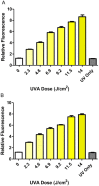
Fig. 1
UVA dose response of DHR123 fluorescence in (A) HaCaT and (B) HDFn cells. Cells loaded with DHR123 were shown to exhibit significantly increased fluorescence intensity over controls at all UVA irradiances (one-way ANOVA including Bonferroni's post-hoc test, P<0.001, _n_=8). Data representative of 2 repeats, _n_=8 replicates for each experimental dose, bars represent means±SEM.
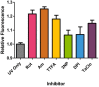
Fig. 2
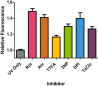
Summary of relative fluorescence intensity of HaCaT cells treated with respiratory chain inhibitors for 18 h prior to exposure of 14 J/cm2 UVA. Significant increases in DHR123 fluorescence intensity over control (i.e. UVA exposure in the absence of inhibitor) were found in rotenone (Rot), antimycin (Am), TTFA and TaClo for 18 h compared to UVA alone (control) (*P<0.05, _n_=8, as analysed by a one-way ANOVA with Dunnett's post-hoc test). No significant difference in fluorescence intensity was found for 3NP and DPI treatment 18 h, (_P_>0.05). Data representative of 2 repeats, _n_=8 replicates for each inhibitor treatment, bars represent means±SEM.
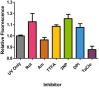
Fig. 3
Summary of relative fluorescence intensity of HDFn cells treated with respiratory chain inhibitors for 18 h before exposure to 14 J/cm2 UVA. Significant increases in DHR123 fluorescence intensity over control (i.e. UVA exposure in the absence of inhibitor) were found in TTFA and 3NP at 18 h treatment compared to UVA alone (control) (*P<0.05, _n_=8, as analysed by a one-way ANOVA with Dunnett's post-hoc test). No significant difference in fluorescence intensity was found for all other inhibitor treatment 18 h, (_P_>0.05). Data representative of 2 repeats, _n_=8 replicates for each inhibitor treatment, bars represent means±SEM.
Fig. 4
Summary of relative fluorescence intensity of HepG2 cells treated with respiratory chain inhibitors for 18 h before exposure to 14 J/cm2 UVA. Significant increases in DHR123 fluorescence intensity over control (i.e. UVA exposure in the absence of inhibitor) were observed for all inhibitors at 18 h treatment compared to UVA alone (control) (*P<0.01, _n_=8, analysed by a one-way ANOVA with Dunnett's post-hoc test). Data representative of 2 repeats, _n_=8 replicates for each inhibitor treatment, bars represent means±SEM.
Similar articles
- Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species.
Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Chen Y, et al. J Cell Sci. 2007 Dec 1;120(Pt 23):4155-66. doi: 10.1242/jcs.011163. J Cell Sci. 2007. PMID: 18032788 - Mitochondrial dysfunction by complex II inhibition delays overall cell cycle progression via reactive oxygen species production.
Byun HO, Kim HY, Lim JJ, Seo YH, Yoon G. Byun HO, et al. J Cell Biochem. 2008 Aug 1;104(5):1747-59. doi: 10.1002/jcb.21741. J Cell Biochem. 2008. PMID: 18395845 - Oxidative stress and ageing.
Birch-Machin MA, Bowman A. Birch-Machin MA, et al. Br J Dermatol. 2016 Oct;175 Suppl 2:26-29. doi: 10.1111/bjd.14906. Br J Dermatol. 2016. PMID: 27667312 Review. - Differential effects of complex II on mitochondrial ROS production and their relation to cardioprotective pre- and postconditioning.
Dröse S. Dröse S. Biochim Biophys Acta. 2013 May;1827(5):578-87. doi: 10.1016/j.bbabio.2013.01.004. Epub 2013 Jan 16. Biochim Biophys Acta. 2013. PMID: 23333272 Review.
Cited by
- Diabetes-Induced Reactive Oxygen Species: Mechanism of Their Generation and Role in Renal Injury.
Fakhruddin S, Alanazi W, Jackson KE. Fakhruddin S, et al. J Diabetes Res. 2017;2017:8379327. doi: 10.1155/2017/8379327. Epub 2017 Jan 9. J Diabetes Res. 2017. PMID: 28164134 Free PMC article. Review. - Mitochondrial Transfusion Improves Mitochondrial Function Through Up-regulation of Mitochondrial Complex II Protein Subunit SDHB in the Hippocampus of Aged Mice.
Adlimoghaddam A, Benson T, Albensi BC. Adlimoghaddam A, et al. Mol Neurobiol. 2022 Oct;59(10):6009-6017. doi: 10.1007/s12035-022-02937-w. Epub 2022 Jul 14. Mol Neurobiol. 2022. PMID: 35834060 Free PMC article. - Cryptotanshinone protects skin cells from ultraviolet radiation-induced photoaging via its antioxidant effect and by reducing mitochondrial dysfunction and inhibiting apoptosis.
Guo K, Liu R, Jing R, Wang L, Li X, Zhang K, Fu M, Ye J, Hu Z, Zhao W, Xu N. Guo K, et al. Front Pharmacol. 2022 Oct 25;13:1036013. doi: 10.3389/fphar.2022.1036013. eCollection 2022. Front Pharmacol. 2022. PMID: 36386220 Free PMC article. - Quantum Sensing of Free Radical Generation in Mitochondria of Human Keratinocytes during UVB Exposure.
Fan S, Lopez Llorens L, Perona Martinez FP, Schirhagl R. Fan S, et al. ACS Sens. 2024 May 24;9(5):2440-2446. doi: 10.1021/acssensors.4c00118. Epub 2024 May 14. ACS Sens. 2024. PMID: 38743437 Free PMC article. - Determination of the Action Spectrum of UVR-Induced Mitochondrial DNA Damage in Human Skin Cells.
Latimer JA, Lloyd JJ, Diffey BL, Matts PJ, Birch-Machin MA. Latimer JA, et al. J Invest Dermatol. 2015 Oct;135(10):2512-2518. doi: 10.1038/jid.2015.194. Epub 2015 Jun 1. J Invest Dermatol. 2015. PMID: 26030182
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources