Effects and mechanisms of 8-prenylnaringenin on osteoblast MC3T3-E1 and osteoclast-like cells RAW264.7 - PubMed (original) (raw)
Effects and mechanisms of 8-prenylnaringenin on osteoblast MC3T3-E1 and osteoclast-like cells RAW264.7
Dan Luo et al. Food Sci Nutr. 2014 Jul.
Abstract
8-Prenylnaringenin (8-PN) is a phytoestrogen with the highest estrogenic activity. The objective of the present study was to confirm the superiority of 8-PN on bone metabolisms and the estrogen receptor (ER) subtype mediating effects of 8-PN. The osteoblast MC3T3-E1 and osteoclast-like cell line RAW264.7 were treated with 17β-estradiol (10(-8) mol/L), genistein (10(-5) mol/L), daidzein (10(-5) mol/L), 8-PN (10(-5) mol/L) alone or in the presence of ERα antagonist MPP (10(-7) mol/L) and ERβ antagonist PTHPP (1.5 × 10(-7) mol/L). It has been found that 8-PN did not affect osteoblast proliferation, and that 8-PN increased alkaline phosphatase (ALP) activity, osteocalcin (OCN) concentrations, and the mineralized nodules. 8-PN inhibited RAW264.7 differentiating into osteoclasts and reduced the pit area of bone resorption. 8-PN could also inhibit the protein and mRNA expression of receptor activator of nuclear factor-κB ligand (RANKL) in osteoblasts, and conversely promote the expression of osteoprotegerin (OPG). These effects of 8-PN were mainly inhibited not by PTHPP but by MPP and they were weaker than estrogen's effects but stronger than those of genistein and daidzein. In conclusion, the effects of 8-PN on promoting osteoblastic bone formation and inhibiting osteoclastic bone resorption were mediated by ERα instead of ERβ and the efficacy was more potent than that of the two classic phytoestrogens: genistein and daidzein.
Keywords: 8-Prenylnaringenin; daidzein; estrogen receptor; genistein; osteoblast; osteoclast.
Figures
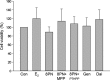
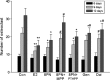
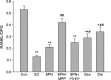
Figure 1
Effects of 48 h treatment of 17_β_-estradiol (E2, 10−8 mol/L), genistein (Gen, 10−5 mol/L), daidzein (Dai, 10−5 mol/L), and 8-prenylnaringenin (8PN, 10−5 mol/L) alone or supplemented with ER_α_ antagonist methyl-piperidino-pyrazole (MPP, 10−7 mol/L) or ER_β_ antagonist 4-[2-phenyl-5,7-bis (tri-fluoro-methyl) pyrazolo [1,5-a] pyrimidin-3-yl] phenol (PTHPP, 1.5 × 10−7 mol/L) on the number of MC3T3-E1 cells. Con, control group; ER, estrogen receptor.
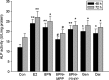
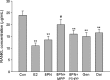
Figure 2
Effects of 48 or 72 h treatment of 17_β_-estradiol (E2, 10−8 mol/L), genistein (Gen, 10−5 mol/L), daidzein (Dai, 10−5 mol/L), and 8-prenylnaringenin (8PN,10−5 mol/L) alone or supplemented with ER_α_ antagonist methyl-piperidino-pyrazole (MPP, 10−7 mol/L) or ER_β_ antagonist 4-[2-phenyl-5,7-bis (tri-fluoro-methyl) pyrazolo [1,5-a] pyrimidin-3-yl] phenol (PTHPP, 1.5 × 10−7 mol/L) on the alkaline (ALP) activity (U/L) of MC3T3-E1. *P < 0.05 and **P < 0.01 versus control group (Con), #P < 0.05 versus 8PN group. ER, estrogen receptor; ALP, alkaline phosphatase.
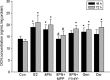
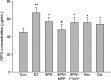
Figure 3
Effects of 48 or 72 h treatment of 17_β_-estradiol (E2, 10−8 mol/L), genistein (Gen, 10−5 mol/L), daidzein (Dai, 10−5 mol/L), and 8-prenylnaringenin (8PN, 10−5 mol/L) alone or supplemented with ER_α_ antagonist methyl-piperidino-pyrazole (MPP, 10−7 mol/L) or ER_β_ antagonist 4-[2-phenyl-5,7-bis (tri-fluoro-methyl) pyrazolo [1,5-a] pyrimidin-3-yl] phenol (PTHPP, 1.5 × 10−7 mol/L) on the osteocalcin (OCN) concentration (ng/mL) of MC3T3-E1. *P < 0.05 versus control group (Con). #P < 0.05 versus 8PN group. ER, estrogen receptor.
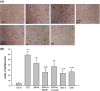
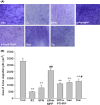
Figure 4
(A) Effects of 17_β_-estradiol (E2, 10−8 mol/L), genistein (Gen, 10−5 mol/L), daidzein (Dai, 10−5 mol/L), and 8-prenylnaringenin (8PN, 10−5 mol/L) alone or supplemented with ER_α_ antagonist methyl-piperidino-pyrazole (MPP, 10−7 mol/L) or ER_β_ antagonist 4-[2-phenyl-5,7-bis (tri-fluoro-methyl) pyrazolo [1,5-a] pyrimidin-3-yl] phenol (PTHPP, 1.5 × 10−7 mol/L) on mineralized nodules of MC3T3-E1 demonstrated by alizarin red staining at day 28 (100×). (B) Different counts of calcified nodes among groups. **P < 0.01 versus control group (Con), #P < 0.05 versus 8PN group. ER, estrogen receptor.
Figure 5
Effects of 17_β_-estradiol (E2, 10−8 mol/L), genistein (Gen, 10−5 mol/L), daidzein (Dai, 10−5 mol/L), and 8-prenylnaringenin (8PN, 10−5 mol/L) alone or supplemented with ER_α_ antagonist methyl-piperidino-pyrazole (MPP, 10−7 mol/L) or ER_β_ antagonist 4-[2-phenyl-5,7-bis (tri-fluoro-methyl) pyrazolo [1,5-a] pyrimidin-3-yl] phenol (PTHPP, 1.5 × 10−7 mol/L) on the number of osteoclast-like cells characterized via tartrate-resistant acid phosphatase (TRAP) staining from RAW264.7 induced by macrophage colony-stimulating factor (M-CSF) and receptor activator for nuclear factor-κ B ligand (RANKL) for 6, 9, 12 days (×200). *P < 0.01 and **P < 0.01 vs. control group (Con), #P < 0.05 versus 8PN group. ER, estrogen receptor.
Figure 6
The characteristics of bone resorption pits on bone slices as shown by arrows (400×).
Figure 7
(A) Effects of 17_β_-estradiol (E2, 10−8 mol/L), genistein (Gen, 10−5 mol/L), daidzein (Dai, 10−5 mol/L), and 8-prenylnaringenin (8PN, 10−5 mol/L) alone or supplemented with ER_α_ antagonist methyl-piperidino-pyrazole (MPP, 10−7 mol/L) or ER_β_ antagonist 4-[2-phenyl-5,7-bis (tri-fluoro- methyl) pyrazolo [1,5-a] pyrimidin-3-yl] phenol (PTHPP, 1.5 × 10−7 mol/L) on bone resorption pits on bone slice observed under optical microscope (100×). (B) Different area of bone resorption pits calculated by the IPP software among groups. **P < 0.01 versus control group (Con), #P < 0.05 and ##P < 0.01 versus 8PN group. ER, estrogen receptor.
Figure 8
Effects of 17_β_-estradiol (E2, 10−8 mol/L), genistein (Gen, 10−5 mol/L), daidzein (Dai, 10−5 mol/L), and 8-prenylnaringenin (8PN, 10−5 mol/L) alone or supplemented with ER_α_ antagonist methyl-piperidino-pyrazole (MPP, 10−7 mol/L) or ER_β_ antagonist 4-[2-phenyl-5,7-bis (tri-fluoro-methyl) pyrazolo [1,5-a] pyrimidin-3-yl] phenol (PTHPP, 1.5 × 10−7 mol/L) on the secretion of receptor activator for nuclear factor-κ B ligand (RANKL) protein in MC3T3-E1 cells. **P < 0.01 versus control group (Con), #P < 0.05 versus 8PN group. ER, estrogen receptor.
Figure 9
Effects of 17_β_-estradiol (E2, 10−8 mol/L), genistein (Gen, 10−5 mol/L), daidzein (Dai, 10−5 mol/L), and 8-prenylnaringenin (8PN, 10−5 mol/L) alone or supplemented with ER_α_ antagonist methyl-piperidino-pyrazole (MPP, 10−7 mol/L) or ER_β_ antagonist 4-[2-phenyl-5,7-bis (tri-fluoro-methyl) pyrazolo [1,5-a] pyrimidin-3-yl] phenol (PTHPP, 1.5 × 10−7 mol/L) on the secretion of osteoprotegerin (OPG) protein in MC3T3-E1 cells. *P < 0.05 and **P < 0.01 versus control group (Con), #P < 0.05 versus 8PN group. ER, estrogen receptor.
Figure 10
Effects of 17_β_-estradiol (E2, 10−8 mol/L), genistein (Gen, 10−5 mol/L), daidzein (Dai, 10−5 mol/L), and 8-prenylnaringenin (8PN, 10−5 mol/L) alone or supplemented with ER_α_ antagonist methyl-piperidino-pyrazole (MPP, 10−7 mol/L) or ER_β_ antagonist 4-[2-phenyl-5,7-bis (tri-fluoro-methyl) pyrazolo [1,5-a] pyrimidin-3-yl] phenol (PTHPP, 1.5 × 10−7 mol/L) on the relative value of secreting protein of receptor activator for nuclear factor-_κ_B ligand (RANKL) to osteoprotegerin (OPG) in MC3T3-E1 cells. *P < 0.05 and **P < 0.01 versus control group (Con), #P < 0.05 and ##P < 0.01 versus 8PN group. ER, estrogen receptor.
Figure 11
Effects of 17_β_-estradiol (E2, 10−8 mol/L), genistein (Gen, 10−5 mol/L), daidzein (Dai, 10−5 mol/L), and 8-prenylnaringenin (8PN, 10−5 mol/L) alone or supplemented with ER_α_ antagonist methyl-piperidino-pyrazole (MPP, 10−7 mol/L) or ER_β_ antagonist 4-[2-phenyl-5,7-bis (tri-fluoro-methyl) pyrazolo [1,5-a] pyrimidin-3-yl] phenol (PTHPP, 1.5 × 10−7 mol/L) on the expression of receptor activator for nuclear factor-_κ_B ligand (RANKL) and osteoprotegerin (OPG) mRNA in MC3T3-E1 cells. ER, estrogen receptor.
Similar articles
- Zinc modifies the effect of phyto-oestrogens on osteoblast and osteoclast differentiation in vitro.
Karieb S, Fox SW. Karieb S, et al. Br J Nutr. 2012 Nov 28;108(10):1736-45. doi: 10.1017/S0007114511007355. Epub 2012 Jan 31. Br J Nutr. 2012. PMID: 22289672 - [Effects of daidzein on steroid receptor coactivator-1 expression in MC3T3-E1 cells and the mechanism].
Wang LF, Wang JF, Jin WF, Wang HF, Zhang SF, Gao JJ. Wang LF, et al. Zhong Xi Yi Jie He Xue Bao. 2011 Nov;9(11):1248-53. doi: 10.3736/jcim20111114. Zhong Xi Yi Jie He Xue Bao. 2011. PMID: 22088592 Chinese. - Increased OPG/RANKL ratio in the conditioned medium of soybean-treated osteoblasts suppresses RANKL-induced osteoclast differentiation.
Park K, Ju WC, Yeo JH, Kim JY, Seo HS, Uchida Y, Cho Y. Park K, et al. Int J Mol Med. 2014 Jan;33(1):178-84. doi: 10.3892/ijmm.2013.1557. Epub 2013 Nov 18. Int J Mol Med. 2014. PMID: 24248634 - The action of GH/IGF-I/IGFBP in osteoblasts and osteoclasts.
Chihara K, Sugimoto T. Chihara K, et al. Horm Res. 1997;48 Suppl 5:45-9. doi: 10.1159/000191328. Horm Res. 1997. PMID: 9434044 Review. - Functions and action mechanisms of flavonoids genistein and icariin in regulating bone remodeling.
Ming LG, Chen KM, Xian CJ. Ming LG, et al. J Cell Physiol. 2013 Mar;228(3):513-21. doi: 10.1002/jcp.24158. J Cell Physiol. 2013. PMID: 22777826 Review.
Cited by
- Bone destruction in chronic otitis media is not mediated by the RANKL pathway or estrogen receptor-alpha.
Heo KW, Noh M, Hur DY, Hong TU, Park SY, Kim WJ. Heo KW, et al. Sci Prog. 2023 Jul-Sep;106(3):368504231199204. doi: 10.1177/00368504231199204. Sci Prog. 2023. PMID: 37697808 Free PMC article. - Overview of RAW264.7 for osteoclastogensis study: Phenotype and stimuli.
Kong L, Smith W, Hao D. Kong L, et al. J Cell Mol Med. 2019 May;23(5):3077-3087. doi: 10.1111/jcmm.14277. Epub 2019 Mar 20. J Cell Mol Med. 2019. PMID: 30892789 Free PMC article. Review. - The Potent Phytoestrogen 8-Prenylnaringenin: A Friend or a Foe?
Pohjanvirta R, Nasri A. Pohjanvirta R, et al. Int J Mol Sci. 2022 Mar 15;23(6):3168. doi: 10.3390/ijms23063168. Int J Mol Sci. 2022. PMID: 35328588 Free PMC article. Review. - Effect of alendronate or 8-prenylnaringenin applied as a single therapy or in combination with vibration on muscle structure and bone healing in ovariectomized rats.
Komrakova M, Rechholtz C, Pohlmann N, Lehmann W, Schilling AF, Wigger R, Sehmisch S, Hoffmann DB. Komrakova M, et al. Bone Rep. 2019 Aug 27;11:100224. doi: 10.1016/j.bonr.2019.100224. eCollection 2019 Dec. Bone Rep. 2019. PMID: 31516917 Free PMC article. - Humulus lupulus Promoting Osteoblast Activity and Bone Integrity: Effects and Mechanisms.
Wanionok NE, Colareda GA, Fernandez JM. Wanionok NE, et al. Biology (Basel). 2025 May 21;14(5):582. doi: 10.3390/biology14050582. Biology (Basel). 2025. PMID: 40427771 Free PMC article.
References
- Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J, Nilsson S, et al. Differential response of estrogen receptor alpha and estrogen receptor beta to partial estrogen agonists/antagonists. Mol. Pharmacol. 1998;54:105–112. - PubMed
- Böttner M, Christoffel J, Wuttke W. Effects of long-term treatment with 8-prenylnaringenin and oral estradiol on the GH-IGF-1 axis and lipid metabolism in rats. J. Endocrinol. 2008;1982:395–401. - PubMed
- Bovee TF, Helsdingen RJ, Rietjens IM, Keijer J, Hoogenboom RL. Rapid yeast estrogen bioassays stably expressing human estrogen receptors alpha and beta, andgreen fluorescent protein: a comparison of different compounds with both receptortypes. J. Steroid Biochem. Mol. Biol. 2004;91:99–109. - PubMed
- Brunelli E, Pinton G, Chianale F, Graziani A, Appendino G, Moro L. 8-Prenylnaringenin inhibits epidermal growth factor-induced MCF-7 breast cancer cell proliferation by targeting phosphatidylinositol-3-OH kinase activity. J. Steroid Biochem. Mol. Biol. 2009;113:163–170. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources