GLP-1 improves neuropathology after murine cold lesion brain trauma - PubMed (original) (raw)
GLP-1 improves neuropathology after murine cold lesion brain trauma
Brian DellaValle et al. Ann Clin Transl Neurol. 2014 Sep.
Erratum in
- Erratum: GLP-1 receptor activation improves neurological outcome after murine brain trauma 1 (9), pp 1-12.
[No authors listed] [No authors listed] Ann Clin Transl Neurol. 2016 Aug 26;3(8):664. doi: 10.1002/acn3.340. eCollection 2016 Aug. Ann Clin Transl Neurol. 2016. PMID: 27606348 Free PMC article.
Abstract
Objectives: In this study, we address a gap in knowledge regarding the therapeutic potential of acute treatment with a glucagon-like peptide-1 (GLP-1) receptor agonist after severe brain trauma. Moreover, it remains still unknown whether GLP-1 treatment activates the protective, anti-neurodegenerative cAMP response element binding protein (CREB) pathway in the brain in vivo, and whether activation leads to observable increases in protective, anti-neurodegenerative proteins. Finally, we report the first use of a highly sensitive in vivo imaging agent to assess reactive species generation after brain trauma.
Methods: Severe trauma was induced with a stereotactic cryo-lesion in mice and thereafter treated with vehicle, liraglutide, or liraglutide + GLP-1 receptor antagonist. A therapeutic window was established and lesion size post-trauma was determined. Reactive oxygen species were visualized in vivo and quantified directly ex vivo. Hematological analysis was performed over time. Necrotic and apoptotic tone and neuroinflammation was assessed over time. CREB activation and CREB-regulated cytoprotective proteins were assessed over time.
Results: Lira treatment reduced lesion size by ∼50% through the GLP-1 receptor. Reactive species generation was reduced by ∼40-60%. Necrotic and apoptotic tone maintained similar to sham in diseased animals with Lira treatment. Phosphorylation of CREB was markedly increased by Lira in a GLP-1 receptor-dependent manner. CREB-regulated cytoprotective and anti-neurodegenerative proteins increased with Lira-driven CREB activation.
Interpretation: These results show that Lira has potent effects after experimental trauma in mice and thus should be considered a candidate for critical care intervention post-injury. Moreover, activation of CREB in the brain by Lira - described for the first time to be dependent on pathology - should be investigated further as a potential mechanism of action in neurodegenerative disorders.
Figures
Figure 1
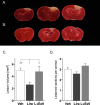
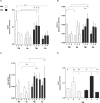
Lesion size determination. Traumatic brain injury (TBI) was induced and thereafter, each animal was randomly assigned to vehicle, liraglutide (Lira), or GLP-1 receptor antagonist, exendin 9-39 (Ex9) + Lira arms. At day 2 post lesion, brains were sliced (1 mm), stained with 1% 2,3,5-triphenyltexstrazolium, and quantified with planimetry. Representative (A) vehicle and (B) Lira-treated injury volume after staining. (C) Bar graph presenting lesion volume (mm3) at day 2 in vehicle (white), Lira (black) and Ex9 + Lira (light gray) treatment groups. (D) Average number of lesioned sections quantified per animal. Adjacent sections that had lesioned tissue which did not pass through the entire 1 mm were not quantified. Sham animal brains were fully stained. Presented as mean + SEM; vehicle and Lira data are the result of three independent experiments (n = 19, 20; no difference between data sets from each experiment (one-way ANOVA: vehicle: P = 0.90; Lira: P = 0.14) and Ex9 + Lira from one experiment (n = 6); *, **P < 0.05, P < 0.01. GLP-1, glucagon-like peptide-1.
Figure 2
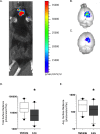
Reactive oxygen and nitrogen species chemiluminescence. Traumatic brain injury (TBI) animals were injected with the sensitive luminol derivative, L-012. L-012 emits chemiluminescence when the molecule reacts with reactive oxygen and nitrogen species. Classical in vivo imaging was not feasible due to the confounding signal from the skin slice (A) and thus imaging was performed at days 1, 2, and 4 post-TBI and the time interval with the highest signal (day 1) was used to quantify reactive species ex vivo. At day 1, brains were immediately excised and analyzed for chemiluminescence photon emission. Representative chemiluminescence signal from (B) vehicle and (C) Lira-treated brains at day 1 post lesion. Box and whiskers plot presenting (D) total, (E) average (per pixel) surface radiance (photons/cm2/sr) from each brain for each treatment arm- vehicle (white) and Lira (black). Sham animal brains did not give a signal above background. Data were normal after log transformation and are presented as median ± 10–90 percentile; n = 10; *P < 0.05.
Figure 3
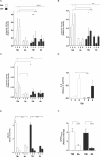
Disease state protein levels. Traumatic brain injury (TBI) animal brains were excised at days 1, 2 and 4 post lesion and probed with antibodies for _α_-spectrin fragments 120 (A), 145 (B), and 150 (C) kDa, (D) IL-6, and (E) albumin leakage to determine protein content relative to housekeeping GAPDH or _β_-tubulin. All antigens were tested for each treatment arm – vehicle (white) or Lira (black) – and with or without TBI. _α_-spectrin bar graphs represent cleavage fragments of varying sizes: 120 kDa (apoptosis), 145 kDa (necrosis), and 150 kDa (necrosis). (F) GFAP content in the plasma was assessed by dot blot to determine GFAP leakage out of the brain at day 1. Data are presented as bar graphs of antigens relative to housekeeping with mean + SEM for normal data (E and F) and median + interquartile range for log-transformed parametric data (A–D); TBI: n = 5–7; Sham: n = 3–6; *, **, ***, ****P < 0.05, 0.01, 0.001, 0.0001.
Figure 4
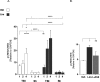
Activation of cAMP response element binding protein. Traumatic brain injury (TBI) animal brains were excised at days 1, 2, and 4 post lesion and probed for CREB, pCREBser133 and GAPDH. normalized to GAPDH. (A) Vehicle (white) or Lira (black)-treated animals were tested with or without TBI on each day. (B) In an independent experiment, animals received vehicle, Lira or exendin 9-39 + Lira immediately after TBI for 2 days post lesion. Each antigen is normalized to GAPDH and subsequently presented as pCREB/CREB ratio. Data were (A) normal after log transformation and (B) in original distribution. Data are presented as bar graphs with (A) median + interquartile range and (B) mean + SEM; TBI and sham: n = 5–7; *, ****P < 0.05, 0.0001.
Figure 5
Cytoprotective protein levels regulated by cAMP response element binding protein. Traumatic brain injury (TBI) animal brains were excised at days 1, 2, and 4 post lesion and probed with antibodies for (A) BDNF, (B) PGC-1_α_, (C) Ngb, and (D) Bcl-2. All antigens were tested for each treatment arm – vehicle (white) or Lira (gray) – and with or without TBI. Data are presented as bar graphs of antigens relative to GAPDH with mean + SEM for normal data (D) and median + interquartile range for log-transformed parametric data (B) and rank analyzed data (A and C); TBI: n = 5–7; Sham: n = 3–6; *, **, ***P < 0.05, 0.01, 0.001.
Figure 6
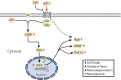
Illustrative summary of results. Data from this study suggest that glucagon-like peptide-1 (GLP-1) agonism through GLP-1 receptors in the brain phosphorylates CREB under pathological conditions associated with cellular trauma. GLP-1 receptor activation can lead to G-protein-coupled receptor activation (G_α_s, G_βγ_) and adenylyl cyclase (AC) conversion of ATP to cyclic AMP (cAMP). Data from previous studies suggest that GLP-1 can increase cAMP and protein kinase A (PKA). CREB-regulated cytoprotective proteins, brain-derived neurotrophic factor, (BDNF), peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC-1_α_), and neuroglobin (Ngb) are upregulated after GLP-1 agonism. Ngb was also upregulated in Lira-treated sham animals despite no CREB activation (dotted line). These molecules can promote an anti-apoptotic/necrotic, antioxidant, anti-neurodegenerative, and pro-neurogenesis environment in affected cells. GLP-1 receptor antagonist, exendin 9-39 (Ex9) blocks activation of CREB and reverses neuroprotective effect on lesion size after TBI. CREB was not activated by Lira treatment in sham animals.
Similar articles
- Oral Administration of Sitagliptin Activates CREB and Is Neuroprotective in Murine Model of Brain Trauma.
DellaValle B, Brix GS, Brock B, Gejl M, Rungby J, Larsen A. DellaValle B, et al. Front Pharmacol. 2016 Dec 1;7:450. doi: 10.3389/fphar.2016.00450. eCollection 2016. Front Pharmacol. 2016. PMID: 27990119 Free PMC article. - Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A.
Oeseburg H, de Boer RA, Buikema H, van der Harst P, van Gilst WH, Silljé HH. Oeseburg H, et al. Arterioscler Thromb Vasc Biol. 2010 Jul;30(7):1407-14. doi: 10.1161/ATVBAHA.110.206425. Epub 2010 May 6. Arterioscler Thromb Vasc Biol. 2010. PMID: 20448207 - Liraglutide attenuates high glucose-induced abnormal cell migration, proliferation, and apoptosis of vascular smooth muscle cells by activating the GLP-1 receptor, and inhibiting ERK1/2 and PI3K/Akt signaling pathways.
Shi L, Ji Y, Jiang X, Zhou L, Xu Y, Li Y, Jiang W, Meng P, Liu X. Shi L, et al. Cardiovasc Diabetol. 2015 Feb 7;14:18. doi: 10.1186/s12933-015-0177-4. Cardiovasc Diabetol. 2015. PMID: 25855361 Free PMC article. - Effects of gamma-glutamyl linker on DPP-IV resistance, duration of action and biological efficacy of acylated glucagon-like peptide-1.
Kerr BD, Flatt PR, Gault VA. Kerr BD, et al. Biochem Pharmacol. 2010 Aug 1;80(3):396-401. doi: 10.1016/j.bcp.2010.04.021. Epub 2010 Apr 22. Biochem Pharmacol. 2010. PMID: 20417187 - Long-acting glucagon-like peptide-1 receptor agonists have direct access to and effects on pro-opiomelanocortin/cocaine- and amphetamine-stimulated transcript neurons in the mouse hypothalamus.
Knudsen LB, Secher A, Hecksher-Sørensen J, Pyke C. Knudsen LB, et al. J Diabetes Investig. 2016 Apr;7 Suppl 1(Suppl 1):56-63. doi: 10.1111/jdi.12463. Epub 2016 Apr 18. J Diabetes Investig. 2016. PMID: 27186357 Free PMC article. Review.
Cited by
- The GLP-1 Receptor Agonist Liraglutide Improves Memory Function and Increases Hippocampal CA1 Neuronal Numbers in a Senescence-Accelerated Mouse Model of Alzheimer's Disease.
Hansen HH, Fabricius K, Barkholt P, Niehoff ML, Morley JE, Jelsing J, Pyke C, Knudsen LB, Farr SA, Vrang N. Hansen HH, et al. J Alzheimers Dis. 2015;46(4):877-88. doi: 10.3233/JAD-143090. J Alzheimers Dis. 2015. PMID: 25869785 Free PMC article. - Treatment With Liraglutide Exerts Neuroprotection After Hypoxic-Ischemic Brain Injury in Neonatal Rats via the PI3K/AKT/GSK3β Pathway.
Zeng SS, Bai JJ, Jiang H, Zhu JJ, Fu CC, He MZ, Zhu JH, Chen SQ, Li PJ, Fu XQ, Lin ZL. Zeng SS, et al. Front Cell Neurosci. 2020 Jan 30;13:585. doi: 10.3389/fncel.2019.00585. eCollection 2019. Front Cell Neurosci. 2020. PMID: 32082121 Free PMC article. - Does Glucagon-like Peptide-1 Ameliorate Oxidative Stress in Diabetes? Evidence Based on Experimental and Clinical Studies.
Petersen KE, Rakipovski G, Raun K, Lykkesfeldt J. Petersen KE, et al. Curr Diabetes Rev. 2016;12(4):331-358. doi: 10.2174/1573399812666150918150608. Curr Diabetes Rev. 2016. PMID: 26381142 Free PMC article. Review. - Oral Administration of Sitagliptin Activates CREB and Is Neuroprotective in Murine Model of Brain Trauma.
DellaValle B, Brix GS, Brock B, Gejl M, Rungby J, Larsen A. DellaValle B, et al. Front Pharmacol. 2016 Dec 1;7:450. doi: 10.3389/fphar.2016.00450. eCollection 2016. Front Pharmacol. 2016. PMID: 27990119 Free PMC article. - The Therapeutic Potential of Glucagon-like Peptide 1 Receptor Agonists in Traumatic Brain Injury.
Harej Hrkać A, Pilipović K, Belančić A, Juretić L, Vitezić D, Mršić-Pelčić J. Harej Hrkać A, et al. Pharmaceuticals (Basel). 2024 Oct 1;17(10):1313. doi: 10.3390/ph17101313. Pharmaceuticals (Basel). 2024. PMID: 39458954 Free PMC article. Review.
References
- Rosenfeld JV, Maas AI, Bragge P, et al. Early management of severe traumatic brain injury. Lancet. 2012;380:1088–1098. - PubMed
- Alahmadi H, Vachhrajani S, Cusimano MD. The natural history of brain contusion: an analysis of radiological and clinical progression. J Neurosurg. 2010;112:1139–1145. - PubMed
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous