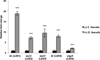The gut commensal Bacteroides thetaiotaomicron exacerbates enteric infection through modification of the metabolic landscape - PubMed (original) (raw)
The gut commensal Bacteroides thetaiotaomicron exacerbates enteric infection through modification of the metabolic landscape
Meredith M Curtis et al. Cell Host Microbe. 2014.
Abstract
The enteric pathogen enterohemorrhagic Escherichia coli (EHEC) causes severe diarrhea, but the influence of the gut microbiota on EHEC infection is largely unknown. A predominant member of the microbiota, Bacteroides thetaiotaomicron (Bt), is resident at EHEC attachment sites. We show that Bt enhances EHEC virulence gene expression through the transcription factor Cra, which is functionally sensitive to sugar concentrations. This enhanced virulence accompanies increased formation of attaching and effacing (AE) lesions requisite for EHEC colonization. Infection with Citrobacter rodentium, a natural mouse pathogen homologous to EHEC, in Bt-reconstituted mice results in increased gut permeability along with exacerbated host pathology and mortality compared to mice deplete of microflora. Bt modifies the metabolite environment at infection sites, increasing metabolites involved in gluconeogenesis, with stark increases in succinate, which can be sensed by Cra. Our findings suggest that microbiota composition affects disease outcome and may explain links between microbiota composition and disease susceptibility.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
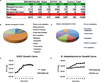
Figure 1. B. thetaiotaomicron (Bt) increases expression of one-fifth of the E. coli array probe sets
A, Summary of microarray results comparing EHEC grown with Bt to EHEC grown alone (EHEC+Bt/EHEC only). B, C, Pie graph categorizing the E. coli genes by function b, that increased ≥ 4-fold or c, that decreased ≥ 2-fold in the presence of Bt D, E, Growth curves (of 6 biological samples, experiments were repeated 2 times, each with 3 independent biological samples) of in vitro growth of D, EHEC or E, Bt. The error bars indicate the standard deviation of the mean. Doubling time for EHEC grown alone and in the presence of Bt is 50 min/gen and 53 min/gen, respectively. Doubling time for Bt grown alone and in the presence of EHEC is 282 min/gen and 97 min/gen, respectively.
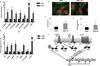
Figure 2. Bt augments EHEC virulence via the catabolite repressor/activator protein
A, qRT-PCR of LEE, stcE and stx2A genes in EHEC grown alone (−) Bt or in the presence of Bt (+) Bt (n= 9; error bars, s.d.; ***P < 0.001, **_P_ < 0.01, *_P_ < 0.05). **B,** Fluorescent actin staining assay of HeLa cells infected with EHEC alone or in the presence of _Bt_, stained with fluorescein isothiocyanate-phalloidin (actin, green) and propidium iodide (bacterial and HeLa DNA, red). Original magnification, 63X. **C,** Quantification of fluorescent actin staining assay of the percentage of HeLa cells infected, as defined by pedestal formation by EHEC. **D,** Number of pedestals/infected cell. (n = 350 cells; error bars, s.d.; *** _P_<0.001). **E,** qRT-PCR of _ler_ in WT, _ΔqseC, ΔqseE, Δcra, ΔkdpE, ΔΔcrakdpE_, and _ΔfusK_ grown alone or in the presence of _Bt_ (n = 9–15; error bars, s.d.; ***_P_ < 0.001, **_P_ < 0.01, _P_ > 0.05 = ns). Each mutant has been normalized to 1 to show the fold-increase of the mutant when grown in the presence of Bt (+) Bt F, Representation of LEE pathogenicity island regulation.
Figure 3. E. faecalis, a member of the Firmicutes phylum, augments EHEC virulence gene expression
qRT-PCR of LEE genes in EHEC grown alone (−) E. faecalis or in the presence of E. faecalis (+) E.faecalis (n= 9; error bars, s.d.; ***P < 0.001).
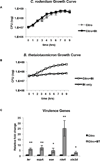
Figure 4. C. rodentium as an EHEC infection model
A, B, Growth curves (of 6 biological samples, experiments were repeated 2 times, each with 3 independent biological samples) of in vitro growth of A, C. rodentium or B, Bt. The error bars indicate the standard deviation of the mean. Doubling time for C. rodentium grown alone and in the presence of Bt is 69 min/gen and 66 min/gen, respectively. Doubling time for Bt grown alone and in the presence of C. rodentium is 282 min/gen and 91 min/gen, respectively. C, qRT-PCR of LEE, nleA and stx genes in C. rodentium grown alone (−) Bt or in the presence of Bt (+) Bt (n = 6; error bars, s.d.; **P < 0.01, * P < 0.05).
Figure 5. Bt mediates its pro-virulence effect on C. rodentium by enhancing expression of the T3SS, not by a bloom in the C. rodentium population
C3H/HeJ mice were treated for 5 days with an antibiotics regimen to deplete gut microbiota. Half of the mice were reconstituted with Bt (+Bt) while the remainder of the mice were left deplete of gut microbiota. Mice were mock-infected (PBS, Bt only) or infected with C. rodentium (Citro, Stx+), C. rodentiumΔstx (CitroΔstx, Stx-), or C. rodentiumΔescN (CitroΔescN). A, Weight loss or gain from baseline (weight at day 0) over the course of infection (blue: mock-infected, reconstituted with Bt; red: _Citro_-infected, deplete of microbiota; green: _Citro_-infected, reconstituted with Bt; purple: _CitroΔstx_-infected, deplete of microbiota; turquoise: _CitroΔstx_-infected, reconstituted with Bt; orange: _CitroΔescN_-infected, deplete of microbiota; light blue: _CitroΔescN_-infected, reconstituted with Bt). B, Survival after infection (n = 7–22 mice/group; error bars, s.d.; ***P < 0.001, **P < 0.01: comparison of Citro vs. Citro+Bt; ##P < 0.01, #P < 0.05: comparison of CitroΔstx vs. CitroΔstx+Bt;+++P < 0.001, ++P < 0.01: comparison of Citro+Bt vs. CitroΔstx+Bt). C, qRT-PCR analysis of ler, espA, eae, and nleA from mRNA isolated from fecal pellets of infected animals. Significance is indicated as follows: one asterisk P≤0.05, two asterisks P≤0.01; three asterisks P≤0.001. D, Ultrastructure of the distal colon harvested five days post-infection from mock-infected (PBS, Bt) or C. rodentium_-in_fected mice either deplete of gut microbiota or reconstituted with Bt, 2500×. Microvilli destruction and C. rodentium forming attaching and effacing (AE) lesions on the colonic epithelium can be observed. Original magnification, 2500X (TEM). E, qRT-PCR of 16s rRNA from the major phylogenetic groups (green: Proteobacteria, blue: Bacteroidetes, purple: Firmicutes) from feces collected on day 1 and day 4 post-infection.
Figure 6. Bt contributes to the accelerated loss of a protective mucosal layer during C. rodentium infection
A, B, Antibiotics-treated C3H/HeJ mice either deplete of gut microbiota or reconstituted with Bt (+Bt) were mock-infected (PBS, Bt only) or infected with C. rodentium (Citro, Stx+), C. rodentiumΔstx (CitroΔstx, Stx-), or C. rodentiumΔescN (CitroΔescN). The histological changes in the colon and cecum were analyzed on day 5 post-infection based on the following scoring system: edema, 0 is no edema and 5 has the highest edema in the submucosa; crypt integrity, 1 = normal, 2 = irregular crypts, 3 = mild crypt loss, 4 = severe crypt loss, 5 = complete crypt loss; neutrophil, neutrophilic infiltration in the wall; apoptosis, number of apoptotic cells per 600× field (n = five fields); bacteria attachment, bacteria associated to the epithelial surface; vasculitis, 0 is no evidence of vasculitis and 5 is the most severe vasculitis. Scoring was performed blindly, and the scores for each parameter are an average of the cecum and distal colon, taken from two independent experiments with 3 mice/experiment. C, Cecum harvested on day 5 post-infection was stained with Muc2 (green) and DAPI (DNA, blue). Original magnification, 10X. D, E, qRTPCR of D, Tff3 (trefoil factor 3) and D, Muc2. RNA was isolated from colonic tissue harvested from mice on day 5 post-infection. (n = 6; error bars, s.d.). F, qRT-PCR of p1411 from feces collected on day 2 post-infection. G, Levels of FITC-Dextran in the serum of mice on day 5 post-infection. Mice were fasted 4 hrs prior to administration of FITC-Dextran via oral gavage. FITC-Dextran levels were determined by measuring fluorescence at excitation 490 nm, emission 525 nm (n = 6; error bars, s.d. ***P < 0.001, **_P_ < 0.01, *_P_ < 0.05, _P_ > 0.05 = ns).
Figure 7. Comparison of metabolic profiles of _Bt_-reconstituted and microflora-deplete animals
A, The expression profiling of metabolites present in the cecum on day 2 post-infection (n = 5). The data are presented as ratios to show fold-changes of the specified metabolite in the individual groups. B, The normalized data were analyzed by Principal Component Analysis (PCA) with unit variance scaling in order to obtain an unbiased overview of the data and to visualize clustering, trends, and outlier metabolites among all groups on the score plots. PCA score plots showed clear separation among the four groups in different colors (yellow: PBS, red: Citro+Bt, blue: Citro, green: Bt), with no outliers detected. The amount of variance in the X matrix explained by PC1 (R2) was 0.732, and estimate of the predictive ability of the model (Q2) (cumulative) was 0.446. C, Absolute levels of succinate, fumarate, and pyruvate present in the cecum on day 2 post-infection. (A.U. Absolute Units; n = 5; error bars, s.d. **P < 0.01, *P < 0.05). D, Protein level of the T3SS translocon protein EspA, encoded within LEE4, secreted into the supernatant of wild-type or Δ_cra_ EHEC cultures grown in the presence of 0.1% glucose, plus increasing levels of succinate. RpoA protein levels in the cell lysate serve as the loading control.
Comment in
- Pathogens' exploitation of the intestinal food web.
Pham N TA, Lawley TD. Pham N TA, et al. Cell Host Microbe. 2014 Dec 10;16(6):703-5. doi: 10.1016/j.chom.2014.11.012. Cell Host Microbe. 2014. PMID: 25498340
Similar articles
- Bacterial Adrenergic Sensors Regulate Virulence of Enteric Pathogens in the Gut.
Moreira CG, Russell R, Mishra AA, Narayanan S, Ritchie JM, Waldor MK, Curtis MM, Winter SE, Weinshenker D, Sperandio V. Moreira CG, et al. mBio. 2016 Jun 7;7(3):e00826-16. doi: 10.1128/mBio.00826-16. mBio. 2016. PMID: 27273829 Free PMC article. - Indole Signaling at the Host-Microbiota-Pathogen Interface.
Kumar A, Sperandio V. Kumar A, et al. mBio. 2019 Jun 4;10(3):e01031-19. doi: 10.1128/mBio.01031-19. mBio. 2019. PMID: 31164470 Free PMC article. - Interactions between Enterohemorrhagic Escherichia coli (EHEC) and Gut Commensals at the Interface of Human Colonoids.
Martins FH, Rajan A, Carter HE, Baniasadi HR, Maresso AW, Sperandio V. Martins FH, et al. mBio. 2022 Jun 28;13(3):e0132122. doi: 10.1128/mbio.01321-22. Epub 2022 May 31. mBio. 2022. PMID: 35638758 Free PMC article. - Citrobacter rodentium: infection, inflammation and the microbiota.
Collins JW, Keeney KM, Crepin VF, Rathinam VA, Fitzgerald KA, Finlay BB, Frankel G. Collins JW, et al. Nat Rev Microbiol. 2014 Sep;12(9):612-23. doi: 10.1038/nrmicro3315. Epub 2014 Aug 4. Nat Rev Microbiol. 2014. PMID: 25088150 Review. - Here, there, and everywhere: How pathogenic Escherichia coli sense and respond to gastrointestinal biogeography.
Woodward SE, Krekhno Z, Finlay BB. Woodward SE, et al. Cell Microbiol. 2019 Nov;21(11):e13107. doi: 10.1111/cmi.13107. Epub 2019 Oct 31. Cell Microbiol. 2019. PMID: 31454133 Review.
Cited by
- The interplay between mitochondria, the gut microbiome and metabolites and their therapeutic potential in primary mitochondrial disease.
Zachos KA, Gamboa JA, Dewji AS, Lee J, Brijbassi S, Andreazza AC. Zachos KA, et al. Front Pharmacol. 2024 Jul 25;15:1428242. doi: 10.3389/fphar.2024.1428242. eCollection 2024. Front Pharmacol. 2024. PMID: 39119601 Free PMC article. Review. - Intratumoral microbiome of adenoid cystic carcinomas and comparison with other head and neck cancers.
Karpinets TV, Mitani Y, Chang CC, Wu X, Song X, Flores II, McDaniel LK, Hoballah YM, Veguilla FJ, Ferrarotto R, Colbert LE, Ajami NJ, Jenq RR, Zhang J, Futreal AP, El-Naggar AK. Karpinets TV, et al. Sci Rep. 2024 Jul 15;14(1):16300. doi: 10.1038/s41598-024-65939-9. Sci Rep. 2024. PMID: 39009605 Free PMC article. - Enterococcus faecalis-derived adenine enhances enterohaemorrhagic Escherichia coli Type 3 Secretion System-dependent virulence.
Martins FH, Rosay T, Rajan A, Carter HE, Turocy T, Mejia A, Crawford JM, Maresso AW, Sperandio V. Martins FH, et al. Nat Microbiol. 2024 Sep;9(9):2448-2461. doi: 10.1038/s41564-024-01747-1. Epub 2024 Jul 4. Nat Microbiol. 2024. PMID: 38965331 - Metabolism of L-arabinose converges with virulence regulation to promote enteric pathogen fitness.
Cottam C, White RT, Beck LC, Stewart CJ, Beatson SA, Lowe EC, Grinter R, Connolly JPR. Cottam C, et al. Nat Commun. 2024 May 25;15(1):4462. doi: 10.1038/s41467-024-48933-7. Nat Commun. 2024. PMID: 38796512 Free PMC article. - IL-22-dependent responses and their role during Citrobacter rodentium infection.
Melchior K, Gerner RR, Hossain S, Nuccio S-P, Moreira CG, Raffatellu M. Melchior K, et al. Infect Immun. 2024 May 7;92(5):e0009924. doi: 10.1128/iai.00099-24. Epub 2024 Apr 1. Infect Immun. 2024. PMID: 38557196 Free PMC article.
References
- Bacchetti De Gregoris T, Aldred N, Clare AS, Burgess JG. Improvement of phylum- and class-specific primers for real-time PCR quantification of bacterial taxa. J Microbiol Methods. 2011;86:351–356. - PubMed
- Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol. 2012;9:88–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI077613/AI/NIAID NIH HHS/United States
- AI077613/AI/NIAID NIH HHS/United States
- T32 AI007520/AI/NIAID NIH HHS/United States
- U01 AI077853/AI/NIAID NIH HHS/United States
- R01 AI105135/AI/NIAID NIH HHS/United States
- AI77853/AI/NIAID NIH HHS/United States
- R37 AI053067/AI/NIAID NIH HHS/United States
- AI053067/AI/NIAID NIH HHS/United States
- R01 CA157996/CA/NCI NIH HHS/United States
- R01 AI053067/AI/NIAID NIH HHS/United States
- 5 T32 AI7520-14/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources