Neurogenesis in the embryonic and adult brain: same regulators, different roles - PubMed (original) (raw)
Review
Neurogenesis in the embryonic and adult brain: same regulators, different roles
Noelia Urbán et al. Front Cell Neurosci. 2014.
Erratum in
- Erratum on: Neurogenesis in the embryonic and adult brain: same regulators, different roles.
Frontiers Production Office. Frontiers Production Office. Front Cell Neurosci. 2015 Apr 23;9:160. doi: 10.3389/fncel.2015.00160. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25954160 Free PMC article.
Abstract
Neurogenesis persists in adult mammals in specific brain areas, known as neurogenic niches. Adult neurogenesis is highly dynamic and is modulated by multiple physiological stimuli and pathological states. There is a strong interest in understanding how this process is regulated, particularly since active neuronal production has been demonstrated in both the hippocampus and the subventricular zone (SVZ) of adult humans. The molecular mechanisms that control neurogenesis have been extensively studied during embryonic development. Therefore, we have a broad knowledge of the intrinsic factors and extracellular signaling pathways driving proliferation and differentiation of embryonic neural precursors. Many of these factors also play important roles during adult neurogenesis, but essential differences exist in the biological responses of neural precursors in the embryonic and adult contexts. Because adult neural stem cells (NSCs) are normally found in a quiescent state, regulatory pathways can affect adult neurogenesis in ways that have no clear counterpart during embryogenesis. BMP signaling, for instance, regulates NSC behavior both during embryonic and adult neurogenesis. However, this pathway maintains stem cell proliferation in the embryo, while it promotes quiescence to prevent stem cell exhaustion in the adult brain. In this review, we will compare and contrast the functions of transcription factors (TFs) and other regulatory molecules in the embryonic brain and in adult neurogenic regions of the adult brain in the mouse, with a special focus on the hippocampal niche and on the regulation of the balance between quiescence and activation of adult NSCs in this region.
Keywords: development of the hippocampus; hippocampal neurogenesis; neural stem cell quiescence; niche signals in adult neurogenesis; regulation of adult neurogenesis.
Figures
Figure 1
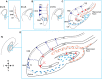
Development of the mouse hippocampus. Schematic representation of the dorsal telencephalon at different embryonic (E) stages and at birth (P0). The indicated area in each picture corresponds to the hippocampal region and is magnified on its right handside (blue squares). (A) At E12.5 the presumptive DNE is located between the HNE and the CH, which produces Cajal-Retzius cells (orange), shown lining the pial side of the cortex. (B) At E14.5 dentate precursors of the primary matrix (dark blue circles) are located in the VZ, and precursor cells start to migrate towards the pial side of the cortex forming the secondary matrix. In the VZ of the HNE, radial glial precursors (depicted in dark blue and triangular body shape) will give rise to hippocampal neurons. (C) At E17.5 the hippocampal fissure is formed and dentate precursor cells migrate to and accumulate there, forming the tertiary matrix (light blue). Cajal-Retzius cells are also present and follow the hippocampal fissure. At this stage the glial scaffold (not shown) extends from the CH to the hippocampal fissure and pial surface, directing the migration of dentate precursor cells. From the HNE, hippocampal neurons (red triangles) are born and migrate along radial glial cells towards their location in the hippocampal fields (CA1 and CA3 are shown). (D) At birth the blades of the DG start to form. Granule neurons in the DG (red triangles) appear first in the upper blade, below the hippocampal fissure. The continuous migration of Cajal-Retzius cells reaches the pial side and promotes the formation of the lower blade of the DG. Precursor cells in the primary and secondary matrix will soon disappear, but cells in the tertiary matrix continue actively dividing and producing granule neurons through postnatal DG development. HNE, hippocampal neuroepithelium; DNE, dentate neuroepithelium; CH, cortical hem; VZ, ventricular zone; 1ry, primary matrix; 2ry, secondary matrix; 3ry, tertiary matrix; DG, dentate gyrus; D, dorsal; M, medial; V, ventral; L, lateral.
Figure 2
Adult neurogenesis in the dentate gyrus. (A) Immunohistochemistry for the neuronal marker NeuN showing the structure of the adult hippocampus. (B) Magnification of the DG region in (A). (C) Graphic representation of the area marked in (B) depicting the neurogenic lineage and several elements of the DG niche. The neurogenic lineage consists of quiescent and active NSCs (including horizontal astrocytes), IPCs (typeIIa, typeIIb), neuroblasts (typeIII) and granule neurons. Neural stem cells and IPCs reside in the SGZ, while neuroblasts and neurons are found in the granule cell layer. Several types of interneurons (red) and astrocytes (purple) are located in different regions of the DG, and together with granule neurons are essential parts of the adult hippocampal niche. Blood vessels throughout the DG and axonal projections in the molecular layer (horizontal lines) also contribute to the regulation of adult neurogenesis at different steps of the lineage.
Figure 3
Niche regulation of mouse adult stem cells in the dentate gyrus. (A) Representation of a neural stem cell (blue) in the adult subgranular zone of the dentate gyrus and some of its interactions with the niche. Granule neurons (yellow), interneurons (red), intermediate precursors (green) and astrocytes (purple) are shown providing quiescence cues, while blood vessels and astrocytes are shown providing activation cues. (B) How quiescence and activation signals are interpreted by adult stem cells is still not known. Here we show several intracellular factors that have been linked to the quiescent (left, Hes5, p. 57, FoxO3 and REST) or active (right, Tlx, Ascl1 and CcnD2) state of stem cells in the adult DG. We also show other factors expressed in NSCs with no clear function in the switch from quiescence to activation (Sox2, Pax6, GFAP and GLAST) in the central part of the schematized cell.
Similar articles
- Persistent Cyfip1 Expression Is Required to Maintain the Adult Subventricular Zone Neurogenic Niche.
Habela CW, Yoon KJ, Kim NS, Taga A, Bell K, Bergles DE, Maragakis NJ, Ming GL, Song H. Habela CW, et al. J Neurosci. 2020 Mar 4;40(10):2015-2024. doi: 10.1523/JNEUROSCI.2249-19.2020. Epub 2020 Jan 27. J Neurosci. 2020. PMID: 31988061 Free PMC article. - Akhirin regulates the proliferation and differentiation of neural stem cells/progenitor cells at neurogenic niches in mouse brain.
Anam MB, Ahmad SAI, Kudo M, Istiaq A, Felemban AAM, Ito N, Ohta K. Anam MB, et al. Dev Growth Differ. 2020 Feb;62(2):97-107. doi: 10.1111/dgd.12646. Epub 2020 Jan 13. Dev Growth Differ. 2020. PMID: 31943155 - Assessing the Role of Ependymal and Vascular Cells as Sources of Extracellular Cues Regulating the Mouse Ventricular-Subventricular Zone Neurogenic Niche.
Quaresima S, Istiaq A, Jono H, Cacci E, Ohta K, Lupo G. Quaresima S, et al. Front Cell Dev Biol. 2022 Apr 5;10:845567. doi: 10.3389/fcell.2022.845567. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35450289 Free PMC article. Review. - Protein S Regulates Neural Stem Cell Quiescence and Neurogenesis.
Zelentsova K, Talmi Z, Abboud-Jarrous G, Sapir T, Capucha T, Nassar M, Burstyn-Cohen T. Zelentsova K, et al. Stem Cells. 2017 Mar;35(3):679-693. doi: 10.1002/stem.2522. Epub 2016 Nov 8. Stem Cells. 2017. PMID: 27753164 - Methods of reactivation and reprogramming of neural stem cells for neural repair.
Tian Z, Zhao Q, Biswas S, Deng W. Tian Z, et al. Methods. 2018 Jan 15;133:3-20. doi: 10.1016/j.ymeth.2017.08.014. Epub 2017 Aug 31. Methods. 2018. PMID: 28864354 Review.
Cited by
- Master regulators of neurogenesis: the dynamic roles of Ephrin receptors across diverse cellular niches.
Rasool D, Jahani-Asl A. Rasool D, et al. Transl Psychiatry. 2024 Nov 6;14(1):462. doi: 10.1038/s41398-024-03168-4. Transl Psychiatry. 2024. PMID: 39505843 Free PMC article. Review. - The Principle of Cortical Development and Evolution.
Yang Z. Yang Z. Neurosci Bull. 2024 Jul 18. doi: 10.1007/s12264-024-01259-2. Online ahead of print. Neurosci Bull. 2024. PMID: 39023844 Review. - Brain organoid as a model to study the role of mitochondria in neurodevelopmental disorders: achievements and weaknesses.
Coronel R, García-Moreno E, Siendones E, Barrero MJ, Martínez-Delgado B, Santos-Ocaña C, Liste I, Cascajo-Almenara MV. Coronel R, et al. Front Cell Neurosci. 2024 Jun 24;18:1403734. doi: 10.3389/fncel.2024.1403734. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38978706 Free PMC article. Review. - Postnatal zinc deficiency due to giardiasis disrupts hippocampal and cerebellar development.
González Maciel A, Rosas López LE, Romero-Velázquez RM, Ramos-Morales A, Ponce-Macotela M, Calderón-Guzmán D, Trujillo-Jiménez F, Alfaro-Rodríguez A, Reynoso-Robles R. González Maciel A, et al. PLoS Negl Trop Dis. 2024 Jul 1;18(7):e0012302. doi: 10.1371/journal.pntd.0012302. eCollection 2024 Jul. PLoS Negl Trop Dis. 2024. PMID: 38950061 Free PMC article. - Effects of crocin on the enhancement of in vitro neurogenesis: Involvement of Notch and CREB/BDNF signaling pathways.
Vafaei S, Mirzaie V, Baghalishahi M, Mousanejad E, Nematollahi-Mahani SN. Vafaei S, et al. Iran J Basic Med Sci. 2024;27(7):914-922. doi: 10.22038/IJBMS.2024.76308.16513. Iran J Basic Med Sci. 2024. PMID: 38800026 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources