Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses - PubMed (original) (raw)
Review
Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses
Bharat B Aggarwal et al. Molecules. 2014.
Abstract
Curcumin (diferuloylmethane), a golden pigment from turmeric, has been linked with antioxidant, anti-inflammatory, anticancer, antiviral, antibacterial, and antidiabetic properties. Most of the these activities have been assigned to methoxy, hydroxyl, α,β-unsaturated carbonyl moiety or to diketone groups present in curcumin. One of the major metabolites of curcumin is tetrahydrocurcumin (THC), which lacks α,β-unsaturated carbonyl moiety and is white in color. Whether THC is superior to curcumin on a molecular level is unclear and thus is the focus of this review. Various studies suggest that curcumin is a more potent antioxidant than THC; curcumin (but not THC) can bind and inhibit numerous targets including DNA (cytosine-5)-methyltransferase-1, heme oxygenase-1, Nrf2, β-catenin, cyclooxygenase-2, NF-kappaB, inducible nitric oxide synthase, nitric oxide, amyloid plaques, reactive oxygen species, vascular endothelial growth factor, cyclin D1, glutathione, P300/CBP, 5-lipoxygenase, cytosolic phospholipase A2, prostaglandin E2, inhibitor of NF-kappaB kinase-1, -2, P38MAPK, p-Tau, tumor necrosis factor-α, forkhead box O3a, CRAC; curcumin can inhibit tumor cell growth and suppress cellular entry of viruses such as influenza A virus and hepatitis C virus much more effectively than THC; curcumin affects membrane mobility; and curcumin is also more effective than THC in suppressing phorbol-ester-induced tumor promotion. Other studies, however, suggest that THC is superior to curcumin for induction of GSH peroxidase, glutathione-S-transferase, NADPH: quinone reductase, and quenching of free radicals. Most studies have indicated that THC exhibits higher antioxidant activity, but curcumin exhibits both pro-oxidant and antioxidant properties.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Figure 1
With increasing pH (alkalinity), curcumin changes to red. Curcumin metabolically converts to tetrahydrocurcumin by using the NADPH-dependent curcumin/dihydrocurcumin reductase (CurA) enzyme. Structurally, curcumin has the α,β-unsaturated carbonyl group, but tetrahydrocurcumin lacks α,β dienes.
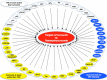
Figure 2
Molecular targets of curcumin vs tetrahydrocurcumin. Curcumin is more effective in modulating some targets, but tetrahydrocurcumin is more effective in others. Some molecules are modulated only by curcumin and not by tetrahydrocurcumin.
Similar articles
- Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism.
Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, Limtrakul P, Badmaev V, Aggarwal BB. Sandur SK, et al. Carcinogenesis. 2007 Aug;28(8):1765-73. doi: 10.1093/carcin/bgm123. Epub 2007 May 23. Carcinogenesis. 2007. PMID: 17522064 - Biological and pharmacological evaluation of dimethoxycurcumin: A metabolically stable curcumin analogue with a promising therapeutic potential.
Teymouri M, Barati N, Pirro M, Sahebkar A. Teymouri M, et al. J Cell Physiol. 2018 Jan;233(1):124-140. doi: 10.1002/jcp.25749. Epub 2017 Mar 31. J Cell Physiol. 2018. PMID: 27996095 Review. - Involvement of the beta-diketone moiety in the antioxidative mechanism of tetrahydrocurcumin.
Sugiyama Y, Kawakishi S, Osawa T. Sugiyama Y, et al. Biochem Pharmacol. 1996 Aug 23;52(4):519-25. doi: 10.1016/0006-2952(96)00302-4. Biochem Pharmacol. 1996. PMID: 8759023 - Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane).
Sandur SK, Ichikawa H, Pandey MK, Kunnumakkara AB, Sung B, Sethi G, Aggarwal BB. Sandur SK, et al. Free Radic Biol Med. 2007 Aug 15;43(4):568-80. doi: 10.1016/j.freeradbiomed.2007.05.009. Epub 2007 May 16. Free Radic Biol Med. 2007. PMID: 17640567 Free PMC article. - Multitargeting by curcumin as revealed by molecular interaction studies.
Gupta SC, Prasad S, Kim JH, Patchva S, Webb LJ, Priyadarsini IK, Aggarwal BB. Gupta SC, et al. Nat Prod Rep. 2011 Nov;28(12):1937-55. doi: 10.1039/c1np00051a. Epub 2011 Oct 6. Nat Prod Rep. 2011. PMID: 21979811 Free PMC article. Review.
Cited by
- The anticancer potential of tetrahydrocurcumin-phytosomes against oral carcinoma progression.
Raouf N, Darwish ZE, Ramadan O, Barakat HS, Elbanna SA, Essawy MM. Raouf N, et al. BMC Oral Health. 2024 Sep 26;24(1):1126. doi: 10.1186/s12903-024-04856-9. BMC Oral Health. 2024. PMID: 39327561 Free PMC article. - Tetrahydrocurcumin Ameliorates Diabetic Cardiomyopathy by Attenuating High Glucose-Induced Oxidative Stress and Fibrosis via Activating the SIRT1 Pathway.
Li K, Zhai M, Jiang L, Song F, Zhang B, Li J, Li H, Li B, Xia L, Xu L, Cao Y, He M, Zhu H, Zhang L, Liang H, Jin Z, Duan W, Wang S. Li K, et al. Oxid Med Cell Longev. 2019 May 9;2019:6746907. doi: 10.1155/2019/6746907. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31210844 Free PMC article. - Short term effect of tetrahydrocurcumin on adipose angiogenesis in very high-fat diet-induced obesity mouse model.
Yoysungnoen B, Srisawat U, Piyabhan P, Duansak N, Sookprasert N, Mathuradavong N, Poomipark N, Munkong N, Tingpej P, Changtam C. Yoysungnoen B, et al. Front Nutr. 2023 Oct 9;10:1221935. doi: 10.3389/fnut.2023.1221935. eCollection 2023. Front Nutr. 2023. PMID: 37876615 Free PMC article. - Biological and therapeutic activities, and anticancer properties of curcumin.
Perrone D, Ardito F, Giannatempo G, Dioguardi M, Troiano G, Lo Russo L, DE Lillo A, Laino L, Lo Muzio L. Perrone D, et al. Exp Ther Med. 2015 Nov;10(5):1615-1623. doi: 10.3892/etm.2015.2749. Epub 2015 Sep 17. Exp Ther Med. 2015. PMID: 26640527 Free PMC article. - Reductive metabolites of curcumin and their therapeutic effects.
Pandey A, Chaturvedi M, Mishra S, Kumar P, Somvanshi P, Chaturvedi R. Pandey A, et al. Heliyon. 2020 Nov 12;6(11):e05469. doi: 10.1016/j.heliyon.2020.e05469. eCollection 2020 Nov. Heliyon. 2020. PMID: 33241148 Free PMC article. Review.
References
- Aggarwal B.B., Sundaram C., Malani N., Ichikawa H. Curcumin: the Indian solid gold. In: Aggarwal B.B., Surh Y.J., Shishodia S., editors. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease. Springer; New York, NY, USA: 2007. pp. 1–75. Advances in Experimental Medicine and Biology, Series Volume 595. - PubMed
- Aggarwal B.B., Kumar A., Bharti A.C. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous