Long Noncoding RNA MALAT1 Promotes Aggressive Renal Cell Carcinoma through Ezh2 and Interacts with miR-205 - PubMed (original) (raw)
Long Noncoding RNA MALAT1 Promotes Aggressive Renal Cell Carcinoma through Ezh2 and Interacts with miR-205
Hiroshi Hirata et al. Cancer Res. 2015.
Abstract
Recently, long noncoding RNAs (lncRNA) have emerged as new gene regulators and prognostic markers in several cancers, including renal cell carcinoma (RCC). In this study, we investigated the contributions of the lncRNA MALAT1 in RCC with a specific focus on its transcriptional regulation and its interactions with Ezh2 and miR-205. We found that MALAT1 expression was higher in human RCC tissues, where it was associated with reduced patient survival. MALAT1 silencing decreased RCC cell proliferation and invasion and increased apoptosis. Mechanistic investigations showed that MALAT1 was transcriptionally activated by c-Fos and that it interacted with Ezh2. After MALAT1 silencing, E-cadherin expression was increased, whereas β-catenin expression was decreased through Ezh2. Reciprocal interaction between MALAT1 and miR-205 was also observed. Lastly, MALAT1 bound Ezh2 and oncogenesis facilitated by MALAT1 was inhibited by Ezh2 depletion, thereby blocking epithelial-mesenchymal transition via E-cadherin recovery and β-catenin downregulation. Overall, our findings illuminate how overexpression of MALAT1 confers an oncogenic function in RCC that may offer a novel theranostic marker in this disease.
©2015 American Association for Cancer Research.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
No potential conflicts of interests were disclosed.
Figures
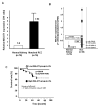
Figure 1. MALAT1 expression and association with clinical parameters in renal cancer tissues
A. Comparison of MALAT1 expression in paired human clinical samples (each normal expression is “1”. B. MALAT1 expression in clinical samples and renal cancer cell lines (786-O, A-498, Caki-1, Caki-2), C. Association of MALAT1 expression with overall survival (Kaplan Meier plot).
Figure 2. Effect of MALAT1 knock down on renal cancer cell function (A-498, 786-O)
Two renal cancer cell lines (A-498 and 786-O) were transiently transfected with either si-MALAT1 (No.1, No.2) or control (si-NC). A. Validation of si-MALAT1 knock down in RCC cell lines, B. Cell viability assay, C. Invasion assay, D. Flow cytometric analysis of apoptosis in si-NC or si-MALAT1 transfected renal cancer cells. Error bars represent ±S.D. (standard deviation).
Figure 3. Transcriptional activation of MALAT1 by c-Fos in 786-O cells
A. c-Fos expression level in renal cancer and matched normal kidney tissues, B. Pearson’s correlation between c-Fos mRNA and MALAT1 mRNA, C. Validation of c-Fos overexpression in 786-O cells after transfection, D. MALAT1 mRNA expression level after transfection of c-Fos overexpressing vector plasmid and control, E. Potential c-Fos binding region in the promoter region of MALAT1 used for construction of luciferase vector containing the binding region, F. luciferase reporter assay (pCMV6-empty vs pCMV6-c-Fos), Error bars represent ±S.D. (standard deviation).
Figure 4. MALAT1 interacts with Ezh2 in renal cancer
A. Schematic diagram illustrating signaling of MALAT1 and its upstream activator and its downstream effectors in RCC. B. Ezh2 mRNA expression in normal kidney and matched renal cancer tissues, C. Pearson’s correlation between MALAT1 mRNA and Ezh2 mRNA, D. High Ezh2 was associated with shorter overall survival of RCC patients, E. Expression of MALAT1 and Ezh2 mRNA in cell lines (HK-2, A-498, 786-O), F. RNA immunoprecipitation (A-498 and 786-O), (F-1) Western blot (input, rabbit IgG, anti-Ezh2 antibody; immunoprecipitation followed by Western blot with Ezh2 antibody), (F-2) qPCR showing MALAT1 is significantly enriched with the Ezh2 antibody compared to IgG (control antibody) in two renal cancer cell lines (A-498 and 786-O)
Figure 5. Downstream Effect of MALAT1 on Ezh2 mediated pathways in renal cancer
A. Western blot results (si-NC vs si-MALAT1), B. Immunohistochemistry of E-Cadherin in renal cancer and normal kidney tissues. C. Association of MALAT1 mRNA with E-Cadherin protein expression, D. Quantification by ChIP real-time PCR of H3K27me3 in the E-Cadherin gene promoter region using si-NC and si-MALAT1 transfected cells (A-498, 786-O). E. Beta-catenin protein expression (Western blot) in the cell nuclear fraction, F. TOPflash luciferase assay
Figure 6. Interaction between MALAT1 and miR-205 in A-498 and 786-O cells
A. miR-205 expression in cell lines (HK-2, A-498, 786-O), B. representation of the miR-205 binding sites in MALAT1 based on miRcode (
http://www.mircode.org/mircode/
), B. Potential miR-205 binding sites in MALAT1 based on miRcode and UCSC Genome Brower. C. MALAT1 knock down and effect on miR-205 expression (qPCR) in RCC cells (A-498 and 786-O), D. Overexpression of miR-205 in A-498 and 786-O transfected cells (qPCR), E. Effect of miR-205 overexpression on MALAT1 in transfected RCC cell lines (A-498 and 786-O)(qPCR)
Similar articles
- The Role of Long Noncoding RNA (lncRNAs) Biomarkers in Renal Cell Carcinoma.
Rysz J, Konecki T, Franczyk B, Ławiński J, Gluba-Brzózka A. Rysz J, et al. Int J Mol Sci. 2022 Dec 30;24(1):643. doi: 10.3390/ijms24010643. Int J Mol Sci. 2022. PMID: 36614082 Free PMC article. Review. - MALAT1 predicts poor survival in osteosarcoma patients and promotes cell metastasis through associating with EZH2.
Huo Y, Li Q, Wang X, Jiao X, Zheng J, Li Z, Pan X. Huo Y, et al. Oncotarget. 2017 Jul 18;8(29):46993-47006. doi: 10.18632/oncotarget.16551. Oncotarget. 2017. PMID: 28388584 Free PMC article. - LncRNA MALAT1 enhances oncogenic activities of EZH2 in castration-resistant prostate cancer.
Wang D, Ding L, Wang L, Zhao Y, Sun Z, Karnes RJ, Zhang J, Huang H. Wang D, et al. Oncotarget. 2015 Dec 1;6(38):41045-55. doi: 10.18632/oncotarget.5728. Oncotarget. 2015. PMID: 26516927 Free PMC article. - Enhancer of zeste homolog 2 expression is associated with metastasis and adverse clinical outcome in clear cell renal cell carcinoma: a comparative study and review of the literature.
Xu B, Abourbih S, Sircar K, Kassouf W, Mansure JJ, Aprikian A, Tanguay S, Brimo F. Xu B, et al. Arch Pathol Lab Med. 2013 Oct;137(10):1326-36. doi: 10.5858/arpa.2012-0525-OA. Arch Pathol Lab Med. 2013. PMID: 24079759 Review.
Cited by
- Knockdown of Long Noncoding RNA PCAT6 Inhibits Proliferation and Invasion in Lung Cancer Cells.
Wan L, Zhang L, Fan K, Cheng ZX, Sun QC, Wang JJ. Wan L, et al. Oncol Res. 2016;24(3):161-70. doi: 10.3727/096504016X14618564639178. Oncol Res. 2016. PMID: 27458097 Free PMC article. - LncRNA MALAT1 facilitates inflammasome activation via epigenetic suppression of Nrf2 in Parkinson's disease.
Cai LJ, Tu L, Huang XM, Huang J, Qiu N, Xie GH, Liao JX, Du W, Zhang YY, Tian JY. Cai LJ, et al. Mol Brain. 2020 Sep 24;13(1):130. doi: 10.1186/s13041-020-00656-8. Mol Brain. 2020. PMID: 32972446 Free PMC article. - The Role of Long Noncoding RNA (lncRNAs) Biomarkers in Renal Cell Carcinoma.
Rysz J, Konecki T, Franczyk B, Ławiński J, Gluba-Brzózka A. Rysz J, et al. Int J Mol Sci. 2022 Dec 30;24(1):643. doi: 10.3390/ijms24010643. Int J Mol Sci. 2022. PMID: 36614082 Free PMC article. Review. - Long Noncoding RNA ANRIL Supports Proliferation of Adult T-Cell Leukemia Cells through Cooperation with EZH2.
Song Z, Wu W, Chen M, Cheng W, Yu J, Fang J, Xu L, Yasunaga JI, Matsuoka M, Zhao T. Song Z, et al. J Virol. 2018 Nov 27;92(24):e00909-18. doi: 10.1128/JVI.00909-18. Print 2018 Dec 15. J Virol. 2018. PMID: 30258009 Free PMC article. - A 44-gene set constructed for predicting the prognosis of clear cell renal cell carcinoma.
Wang Y, Wang Y, Liu F. Wang Y, et al. Int J Mol Med. 2018 Dec;42(6):3105-3114. doi: 10.3892/ijmm.2018.3899. Epub 2018 Sep 26. Int J Mol Med. 2018. PMID: 30272265 Free PMC article.
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. - PubMed
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics. 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01CA160079/CA/NCI NIH HHS/United States
- I01 BX001123/BX/BLRD VA/United States
- R01CA130860/CA/NCI NIH HHS/United States
- R01 CA160079/CA/NCI NIH HHS/United States
- R01 CA130860/CA/NCI NIH HHS/United States
- R01 CA199694/CA/NCI NIH HHS/United States
- R01 CA138642/CA/NCI NIH HHS/United States
- R01CA138642/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical