Epithelial-mesenchymal transition is regulated at post-transcriptional levels by transforming growth factor-β signaling during tumor progression - PubMed (original) (raw)
Review
Epithelial-mesenchymal transition is regulated at post-transcriptional levels by transforming growth factor-β signaling during tumor progression
Masao Saitoh. Cancer Sci. 2015 May.
Abstract
Transforming growth factor (TGF)-β acts as a tumor suppressor during cancer initiation, but as a tumor promoter during tumor progression. It has become increasingly clear that TGF-β plays fundamental roles in multiple steps of tumor progression, including epithelial-mesenchymal transition (EMT). The EMT, first described by developmental biologists at the beginning of the 1980s, plays crucial roles in appropriate embryonic development, but also functions in adults during wound healing, organ fibrosis, and tumor progression. During EMT, epithelial cells lose their epithelial polarity and acquire mesenchymal phenotypes, endowing them with migratory and invasive properties. Many secreted polypeptides are implicated in this process, and act in a sequential or cooperative manner. TGF-β induces EMT by propagating intracellular signaling pathways and activating transcriptional factors. Here, I discuss new insights into the molecular mechanisms underlying induction of EMT by TGF-β in cooperation with Ras or growth factors, along with the signals that induce EMT through transcriptional and post-transcriptional regulation.
Keywords: Cancer biology; EMT; Smad; TGF-β; signal transduction.
© 2015 The Authors. Cancer Science published by Wiley Publishing Asia Pty Ltd on behalf of Japanese Cancer Association.
Figures
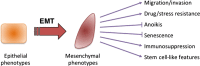
Fig 1
Features of epithelial–mesenchymal transition (EMT).
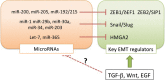
Fig 2
Induction of the epithelial–mesenchymal transition (EMT) by transforming growth factor (TGF)-β through transcriptional regulation of miRNAs and transcription factors. TGF-β directly or indirectly upregulates the key EMT regulators at the transcriptional level, and appears to modulate expression of miRNAs that target the key EMT regulators. δEF1, δ-crystallin/E2-box factor 1; EGF, epidermal growth factor; HMGA2, high mobility group AT-hook 2; ZEB1, zinc finger E-box binding homeobox 1.
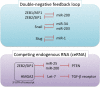
Fig 3
Schematic illustrations of a double-negative feedback loop and the ceRNA pathway. Expression levels of the key EMT regulators and miRNAs suppress each other. Some of the key EMT regulators function as ceRNAs. δEF1, δ-crystallin/E2-box factor 1; HMGA2, high mobility group AT-hook 2; PTEN, phosphatase and tensin homolog; SIP1, Smad-interacting protein 1; TGF-β, transforming growth factor-β; ZEB1, zinc finger E-box binding homeobox 1.
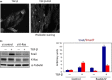
Fig 4
EMT by TGF-β in cooperation with FGF-2 or oncogenic K-Ras. (a) Treatment with both TGF-β and FGF-2 dramatically enhanced epithelial–mesenchymal transition, as determined by phalloidin staining. (b) When K-Ras was knocked down with the siRNA in Panc-1 cells harboring an endogenous oncogenic K-Ras mutation, TGF-β-induced Snail expression was reduced. (c) When RasG12V was transfected into HeLa cells harboring wild-type Ras, Snail (shown in blue) was synergistically upregulated by TGF-β, whereas Smad7 (shown in red), a representative target gene of TGF-β, was slightly inhibited.
Fig 5
Schematic illustrations of epithelial–mesenchymal transition (EMT) regulated by transcriptional and post-transcriptional mechanisms. The key EMT regulators decrease expression of epithelial splicing regulatory proteins (ESRPs), leading to changes in alternative splicing events. FGFR, fibroblast growth factor receptor; FSP, fibroblast-specific protein; SMA, smooth muscle α-actin; ZEB, zinc finger E-box binding homeobox.
Similar articles
- Transcriptional and post-transcriptional regulation in TGF-β-mediated epithelial-mesenchymal transition.
Saitoh M, Miyazawa K. Saitoh M, et al. J Biochem. 2012 Jun;151(6):563-71. doi: 10.1093/jb/mvs040. Epub 2012 Apr 23. J Biochem. 2012. PMID: 22528665 Review. - TGF-β signaling and epithelial-mesenchymal transition in cancer progression.
Katsuno Y, Lamouille S, Derynck R. Katsuno Y, et al. Curr Opin Oncol. 2013 Jan;25(1):76-84. doi: 10.1097/CCO.0b013e32835b6371. Curr Opin Oncol. 2013. PMID: 23197193 Review. - TGF-β orchestrates fibrogenic and developmental EMTs via the RAS effector RREB1.
Su J, Morgani SM, David CJ, Wang Q, Er EE, Huang YH, Basnet H, Zou Y, Shu W, Soni RK, Hendrickson RC, Hadjantonakis AK, Massagué J. Su J, et al. Nature. 2020 Jan;577(7791):566-571. doi: 10.1038/s41586-019-1897-5. Epub 2020 Jan 8. Nature. 2020. PMID: 31915377 Free PMC article. - TGF-β-induced epithelial-mesenchymal transition: a link between cancer and inflammation.
Fuxe J, Karlsson MC. Fuxe J, et al. Semin Cancer Biol. 2012 Oct;22(5-6):455-61. doi: 10.1016/j.semcancer.2012.05.004. Epub 2012 May 22. Semin Cancer Biol. 2012. PMID: 22627188 Review. - Thyroid transcription factor-1 inhibits transforming growth factor-beta-mediated epithelial-to-mesenchymal transition in lung adenocarcinoma cells.
Saito RA, Watabe T, Horiguchi K, Kohyama T, Saitoh M, Nagase T, Miyazono K. Saito RA, et al. Cancer Res. 2009 Apr 1;69(7):2783-91. doi: 10.1158/0008-5472.CAN-08-3490. Epub 2009 Mar 17. Cancer Res. 2009. PMID: 19293183
Cited by
- Factors Determining Epithelial-Mesenchymal Transition in Cancer Progression.
Tomecka P, Kunachowicz D, Górczyńska J, Gebuza M, Kuźnicki J, Skinderowicz K, Choromańska A. Tomecka P, et al. Int J Mol Sci. 2024 Aug 17;25(16):8972. doi: 10.3390/ijms25168972. Int J Mol Sci. 2024. PMID: 39201656 Free PMC article. Review. - Sarcomatoid carcinoma of the pancreas (Review).
Ma Y, Yang Y, Zhang H, Mugaanyi J, Hu Y, Wu S, Lu C, Mao S, Wang K. Ma Y, et al. Oncol Lett. 2024 Aug 5;28(4):477. doi: 10.3892/ol.2024.14610. eCollection 2024 Oct. Oncol Lett. 2024. PMID: 39161336 Free PMC article. Review. - MiR-338-5p, a novel metastasis-related miRNA, inhibits triple-negative breast cancer progression by targeting the ETS1/NOTCH1 axis.
Chen WJ, Ye QQ, Wu HT, Wu Z, Lan YZ, Fang ZX, Lin WT, Liu J. Chen WJ, et al. Heliyon. 2024 Jul 20;10(15):e34949. doi: 10.1016/j.heliyon.2024.e34949. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39157351 Free PMC article. - Nebivolol ameliorates sepsis-evoked kidney dysfunction by targeting oxidative stress and TGF-β/Smad/p53 pathway.
Sabra RT, Bekhit AA, Sabra NT, Abd El-Moeze NA, Fathy M. Sabra RT, et al. Sci Rep. 2024 Jun 26;14(1):14735. doi: 10.1038/s41598-024-64577-5. Sci Rep. 2024. PMID: 38926458 Free PMC article. - Circ6834 suppresses non-small cell lung cancer progression by destabilizing ANHAK and regulating miR-873-5p/TXNIP axis.
Wang M, Ding X, Fang X, Xu J, Chen Y, Qian Y, Zhang J, Yu D, Zhang X, Ma X, Zhu T, Gu J, Zhang X. Wang M, et al. Mol Cancer. 2024 Jun 18;23(1):128. doi: 10.1186/s12943-024-02038-3. Mol Cancer. 2024. PMID: 38890620 Free PMC article.
References
- Derynck R, Miyazono K. The TGF-ß Family. Long Island, New York: Cold Spring Harbor Laboratory Press; 2008.
- Massague J, Blain SW, Lo RS. TGFβ signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. - PubMed
- Wrana JL, Attisano L. The Smad pathway. Cytokine Growth Factor Rev. 2000;11:5–13. - PubMed
- Massague J. Integration of Smad and MAPK pathways: a link and a linker revisited. Genes Dev. 2003;17:2993–7. - PubMed
- Kamaraju AK, Roberts AB. Role of Rho/ROCK and p38 MAP kinase pathways in transforming growth factor-β-mediated Smad-dependent growth inhibition of human breast carcinoma cells in vivo. J Biol Chem. 2005;280:1024–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources