Unequivocal glycyrrhizin isomer determination and comparative in vitro bioactivities of root extracts in four Glycyrrhiza species - PubMed (original) (raw)
Unequivocal glycyrrhizin isomer determination and comparative in vitro bioactivities of root extracts in four Glycyrrhiza species
Mohamed A Farag et al. J Adv Res. 2015 Jan.
Abstract
Glycyrrhiza glabra, commonly known as licorice, is a popular herbal supplement used for the treatment of chronic inflammatory conditions and as sweetener in the food industry. This species contains a myriad of phytochemicals including the major saponin glycoside glycyrrhizin (G) of Glycyrrhetinic acid (GA) aglycone. In this study, 2D-ROESY NMR technique was successfully applied for distinguishing 18α and 18β glycyrrhetinic acid (GA). ROESY spectra acquired from G. glabra, Glycyrrhiza uralensis and Glycyrrhiza inflata crude extracts revealed the presence of G in its β-form. Anti-inflammatory activity of four Glycyrrhiza species, G, glabra, G. uralensis, G. inflata, and G. echinata roots was assessed against COX-1 inhibition revealing that phenolics rather than glycyrrhizin are biologically active in this assay. G. inflata exhibits a strong cytotoxic effect against PC3 and HT29 cells lines, whereas other species are inactive. This study presents an effective NMR method for G isomer assignment in licorice extracts that does not require any preliminary chromatography or any other purification step.
Keywords: G. glabra; G. inflata; G. uralensis; Glycyrrhizin; Licorice; ROESY.
Figures
Graphical abstract
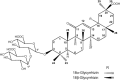
Fig. 1
Chemical structures of _α_- and _β_-glycyrrhizin. Note the carbon numbering system for each compound is used throughout the manuscript for NMR assignment.
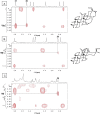
Fig. 2
Identification of β-glycyrrhizin in _G. uralensi_s crude extract by comparison with the 2D ROESY spectra of α- and β-glycyrrhetic acid. Expansions of 2D ROESY spectra for α-glycyrrhetic acid (A), β-glycyrrhetic acid (B), and G. uralensis extract (C) showing correlations through space between H-18 and CH3-28 in the β-isomers but H-18 and CH3-27 as well as CH3-29 in the α-isomer; arrows point to correlations.
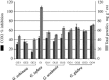
Fig. 3
Anti-inflammatory activity against COX1 and total phenolics (TP) content of Glycyrrhiza sp. extract tested at a dose of 100 μg ml−1 (n = 3). Results are expressed for anti-inflammatory activity as % inhibition to control on the Y1 axis and for polyphenol content as mg g−1 as displayed on the Y2 axis. Glycyrrhiza samples include: (GE1, GE2, GE3; G. echinata), (GU1, GU2, GU3; G. uralensis), (GG1, GG2, GG3, GG4; G. glabra) and (GI; G. inflata), for sample codes, see Table 1.
Similar articles
- Constituent properties of licorices derived from Glycyrrhiza uralensis, G. glabra, or G. inflata identified by genetic information.
Kondo K, Shiba M, Nakamura R, Morota T, Shoyama Y. Kondo K, et al. Biol Pharm Bull. 2007 Jul;30(7):1271-7. doi: 10.1248/bpb.30.1271. Biol Pharm Bull. 2007. PMID: 17603166 - Comparative metabolite profiling and fingerprinting of medicinal licorice roots using a multiplex approach of GC-MS, LC-MS and 1D NMR techniques.
Farag MA, Porzel A, Wessjohann LA. Farag MA, et al. Phytochemistry. 2012 Apr;76:60-72. doi: 10.1016/j.phytochem.2011.12.010. Epub 2012 Feb 13. Phytochemistry. 2012. PMID: 22336263 - Toxicological Effects of Glycyrrhiza glabra (Licorice): A Review.
Nazari S, Rameshrad M, Hosseinzadeh H. Nazari S, et al. Phytother Res. 2017 Nov;31(11):1635-1650. doi: 10.1002/ptr.5893. Epub 2017 Aug 18. Phytother Res. 2017. PMID: 28833680 Review. - A Comprehensive Review for Phytochemical, Pharmacological, and Biosynthesis Studies on Glycyrrhiza spp.
Wang C, Chen L, Xu C, Shi J, Chen S, Tan M, Chen J, Zou L, Chen C, Liu Z, Liu X. Wang C, et al. Am J Chin Med. 2020;48(1):17-45. doi: 10.1142/S0192415X20500020. Epub 2020 Jan 14. Am J Chin Med. 2020. PMID: 31931596 Review.
Cited by
- Two dimensional NMR spectroscopic approaches for exploring plant metabolome: A review.
Mahrous EA, Farag MA. Mahrous EA, et al. J Adv Res. 2015 Jan;6(1):3-15. doi: 10.1016/j.jare.2014.10.003. Epub 2014 Oct 18. J Adv Res. 2015. PMID: 25685540 Free PMC article. Review. - Halophyte Plants as Potential Sources of Anticancer Agents: A Comprehensive Review.
Custodio L, Garcia-Caparros P, Pereira CG, Castelo-Branco P. Custodio L, et al. Pharmaceutics. 2022 Nov 8;14(11):2406. doi: 10.3390/pharmaceutics14112406. Pharmaceutics. 2022. PMID: 36365224 Free PMC article. Review. - Microparticle-enhanced Chaetomium globosum DX-THS3 β-d-glucuronidase production by controlled fungal morphology in submerged fermentation.
Du L, Gao B, Liang J, Wang Y, Xiao Y, Zhu D. Du L, et al. 3 Biotech. 2020 Mar;10(3):100. doi: 10.1007/s13205-020-2068-y. Epub 2020 Feb 6. 3 Biotech. 2020. PMID: 32099741 Free PMC article. - Traditional Chinese medicine Zhusha Anshen Wan: protective effects on liver, kidney, and intestine of the individual drugs using 1H NMR metabolomics.
Wang D, Yu C, Liu B, Wang H. Wang D, et al. Front Pharmacol. 2024 Jan 29;15:1353325. doi: 10.3389/fphar.2024.1353325. eCollection 2024. Front Pharmacol. 2024. PMID: 38370476 Free PMC article. - Two responses to MeJA induction of R2R3-MYB transcription factors regulate flavonoid accumulation in Glycyrrhiza uralensis Fisch.
Li Y, Chen X, Wang J, Zou G, Wang L, Li X. Li Y, et al. PLoS One. 2020 Jul 30;15(7):e0236565. doi: 10.1371/journal.pone.0236565. eCollection 2020. PLoS One. 2020. PMID: 32730299 Free PMC article.
References
- Tanaka A., Horiuchi M., Umano K., Shibamoto T. Antioxidant and anti-inflammatory activities of water distillate and its dichloromethane extract from licorice root (Glycyrrhiza uralensis) and chemical composition of dichloromethane extract. J Sci Food Agric. 2008;88:1158–1165.
- Lee C.K., Park K.K., Lim S.S., Park J.H.Y., Chung W.Y. Effects of the licorice extract against tumor growth and cisplatin-induced toxicity in a mouse xenograft model of colon cancer. Biol Pharm Bull. 2007;30:2191–2195. - PubMed
- Kobayashi M., Fujita K., Katakura T., Utsunomiya T., Pollard R.B., Suzuki F. Inhibitory effect of glycyrrhizin on experimental pulmonary metastasis in mice inoculated with B16 melanoma. Anticancer Res. 2002;22:4053–4058. - PubMed
- Csuk R., Schwarz S., Kluge R., Stroehl D. Synthesis and biological activity of some antitumor active derivatives from glycyrrhetinic acid. Eur J Med Chem. 2010;45:5718–5723. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources