Erlotinib-cisplatin combination inhibits growth and angiogenesis through c-MYC and HIF-1α in EGFR-mutated lung cancer in vitro and in vivo - PubMed (original) (raw)
Erlotinib-cisplatin combination inhibits growth and angiogenesis through c-MYC and HIF-1α in EGFR-mutated lung cancer in vitro and in vivo
Jasmine G Lee et al. Neoplasia. 2015 Feb.
Abstract
Combination treatment for non-small cell lung cancer (NSCLC) is becoming more popular due to the anticipation that it may be more effective than single drug treatment. In addition, there are efforts to genetically screen patients for specific mutations in light of attempting to administer specific anticancer agents that are most effective. In this study, we evaluate the anticancer and anti-angiogenic effects of low dose erlotinib-cisplatin combination in NSCLC in vitro and in vivo. In NSCLC cells harboring epidermal growth factor receptor (EGFR) mutations, combination erlotinib-cisplatin treatment led to synergistic cell death, but there was minimal efficacy in NSCLC cells with wild-type EGFR. In xenograft models, combination treatment also demonstrated greater inhibition of tumor growth compared to individual treatment. The anti-tumor effect observed was secondary to the targeting of angiogenesis, evidenced by decreased vascular endothelial growth factor (VEGF) levels and decreased levels of CD31 and microvessel density. Combination treatment targets angiogenesis through down-regulation of the c-MYC/hypoxia inducible factor 1-alpha (HIF-1α) pathway. In fact, cell lines with EGFR exon 19 deletions expressed high basal levels of c-MYC and HIF-1α and correlate with robust responses to combination treatment. These results suggest that low dose erlotinib-cisplatin combination exhibits its anti-tumor activity by targeting angiogenesis through the modulation of the c-MYC/HIF-1α/VEGF pathway in NSCLC with EGFR exon 19 deletions. These findings may have significant clinical implications in patients with tumors harboring EGFR exon 19 deletions as they may be particularly sensitive to this regimen.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
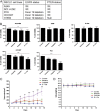
Erlotinib-cisplatin was most effective in cells harboring EGFR exon19 deletion in vitro and in vivo. (A) A report of the EGFR and PTEN status of multiple NSCLC cell lines is shown. (B) Cell viability was quantified with Alamar Blue Assay, and the control group was set to 100% as the standard group. Two-day combination treatment led to synergistic cell death in PC9 and HCC827 cell lines (P < .0001; CI = 0.45 and P = .0005; CI = 0.98, respectively). Combination treatment was not effective in A549, H292, and H1650 cell lines. (C) In the treatment of tumors in xenograft models, there was a significant difference in tumor volume starting at day 5. Combination treatment resulted in the smallest mean tumor volume throughout the treatment course and at the end of the treatment course (day 15) when compared to control, erlotinib alone, and cisplatin alone treatment groups (P = .001, P = .003, and P = .027, respectively). (D) All four groups had similar body weight indicating minimal toxicity. For (C) and (D), specific mean tumor volume and body weights are shown at indicated time points (± SD, n = 4-5 mice each group); * indicates a P value less than .05, ** indicates a P value less than .01, *** indicates a P value less than .001, and **** indicates a P value less than .0001.
Figure 2
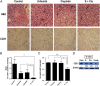
Erlotinib-cisplatin combination treatment led to a significant decrease in tumor vascularity in xenograft mice. (A) H&E staining of tumors demonstrated the least amount of vasculature in the combination group. (B) MVD was quantified in tumor samples stained with CD31 (using DAB). The combination group showed the lowest MVD (0.2%) compared to control (2.8%), erlotinib alone (1.1%), and cisplatin alone (1.2%) treated groups. (C) In vivo angiogenesis was performed by Matrigel plug assay. The Matrigel plug with combination drugs had the lowest hemoglobin contents (0.41) compared to Matrigel plug with control (0.63), erlotinib alone (0.63), and cisplatin alone (0.61). (D) CD31 levels from tumor samples were analyzed through Western blot analysis. Compared to tumors from control (P = .007), erlotinib (P = .04), and cisplatin (P = .01) groups, significantly lower CD31 levels were measured in the combination treatment group. The numerical density of CD31 is indicated below the bands; * indicates a P value less than .05, ** indicates a P value less than .01, *** indicates a P value less than .001, and **** indicates a P value less than .0001.
Figure 3
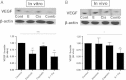
Erlotinib-cisplatin combination treatment led to decreased VEGF levels in vitro and in vivo. (A) The changes in VEGF levels in PC9 cells were quantified through Western blot analysis. There were significantly lower VEGF levels in combination-treated cells among four treatment groups (P = .004). (B) Similarly, through Western blot, combination treatment had the most profound decrease in VEGF levels and was significantly lower than control (P = .003), erlotinib alone (P = .041), and cisplatin alone (P = .024); * indicates a P value less than .05, ** indicates a P value less than .01, *** indicates a P value less than .001, and **** indicates a P value less than .0001.
Figure 4
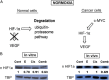
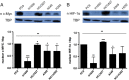
HIF-1α–VEGF pathway was markedly inhibited by combination treatment as demonstrated in vitro and in vivo. (A) A simplified diagram describing the regulation of the HIF-1α and VEGF pathway in normal and cancer cells is shown. (B) Nuclear HIF-1α protein levels were measured with Western blot. Erlotinib-cisplatin combination led to a significant down-regulation of nuclear HIF-1α levels in both PC9 cell line and xenograft mouse models, while individual drug treatment was minimally effective. The numerical density of nuclear HIF-1α is indicated below the bands.
Figure 5
Erlotinib-cisplatin treatment led to a significant decrease in nuclear c-MYC in PC9 cells and xenograft tumors. (A) Combination treatment resulted in the greatest inhibition of c-MYC both in vitro and in vivo. (B) Immunofluorescence staining of c-MYC in PC9 cells and tumor samples further delineates and demonstrates the differences in c-MYC levels among treatment groups. Significantly less green fluorescence is observed in combination-treated groups in vitro and in vivo compared to three other groups (blue, DAPI; green, c-MYC).
Figure 6
High basal levels of c-MYC and HIF-1α were correlated with greater response to treatment. (A) Western blot was performed to measure the basal level of c-MYC in NSCLC cell lines (shown in Figure 1_A_). PC9 and HCC827 (P = .0002) had the highest basal level of c-MYC, followed by A549 (P = .050), H292 (P = .080), and H1650 (P = .007). (B) HCC827 (P = .220) and PC9 also had the highest basal levels of HIF-1α, followed by H292 (P = .045), A549 (P = .050), and H1650 (P = .0002); * indicates a P value less than .05, ** indicates a P value less than .01, *** indicates a P value less than .001, and **** indicates a P value less than .0001.
Similar articles
- Celecoxib-erlotinib combination delays growth and inhibits angiogenesis in EGFR-mutated lung cancer.
Li YX, Wang JL, Gao M, Tang H, Gui R, Fu YF. Li YX, et al. Am J Cancer Res. 2016 Jul 1;6(7):1494-510. eCollection 2016. Am J Cancer Res. 2016. PMID: 27508092 Free PMC article. - Curcumin lowers erlotinib resistance in non-small cell lung carcinoma cells with mutated EGF receptor.
Li S, Liu Z, Zhu F, Fan X, Wu X, Zhao H, Jiang L. Li S, et al. Oncol Res. 2013;21(3):137-44. doi: 10.3727/096504013X13832473330032. Oncol Res. 2013. PMID: 24512728 - Impact of bevacizumab in combination with erlotinib on EGFR-mutated non-small cell lung cancer xenograft models with T790M mutation or MET amplification.
Furugaki K, Fukumura J, Iwai T, Yorozu K, Kurasawa M, Yanagisawa M, Moriya Y, Yamamoto K, Suda K, Mizuuchi H, Mitsudomi T, Harada N. Furugaki K, et al. Int J Cancer. 2016 Feb 15;138(4):1024-32. doi: 10.1002/ijc.29848. Epub 2015 Oct 13. Int J Cancer. 2016. PMID: 26370161 - Interactions between hypoxia and epidermal growth factor receptor in non-small-cell lung cancer.
Swinson DE, O'Byrne KJ. Swinson DE, et al. Clin Lung Cancer. 2006 Jan;7(4):250-6. doi: 10.3816/CLC.2006.n.002. Clin Lung Cancer. 2006. PMID: 16512978 Review. - The intriguing interplay between therapies targeting the epidermal growth factor receptor, the hypoxic microenvironment and hypoxia-inducible factors.
Wouters A, Boeckx C, Vermorken JB, Van den Weyngaert D, Peeters M, Lardon F. Wouters A, et al. Curr Pharm Des. 2013;19(5):907-17. Curr Pharm Des. 2013. PMID: 22973959 Review.
Cited by
- Tetrandrine suppresses lung cancer growth and induces apoptosis, potentially via the VEGF/HIF-1α/ICAM-1 signaling pathway.
Chen Z, Zhao L, Zhao F, Yang G, Wang JJ. Chen Z, et al. Oncol Lett. 2018 May;15(5):7433-7437. doi: 10.3892/ol.2018.8190. Epub 2018 Mar 7. Oncol Lett. 2018. PMID: 29849794 Free PMC article. - Celecoxib-erlotinib combination delays growth and inhibits angiogenesis in EGFR-mutated lung cancer.
Li YX, Wang JL, Gao M, Tang H, Gui R, Fu YF. Li YX, et al. Am J Cancer Res. 2016 Jul 1;6(7):1494-510. eCollection 2016. Am J Cancer Res. 2016. PMID: 27508092 Free PMC article. - Value of combining serum carcinoembryonic antigen and PET/CT in predicting EGFR mutation in non-small cell lung cancer.
Gu J, Xu S, Huang L, Li S, Wu J, Xu J, Feng J, Liu B, Zhou Y. Gu J, et al. J Thorac Dis. 2018 Feb;10(2):723-731. doi: 10.21037/jtd.2017.12.143. J Thorac Dis. 2018. PMID: 29607142 Free PMC article. - Angiogenic activities are increased via upregulation of HIF-1α expression in gefitinib-resistant non-small cell lung carcinoma cells.
Cha JE, Bae WY, Choi JS, Lee SH, Jeong JW. Cha JE, et al. Oncol Lett. 2021 Sep;22(3):671. doi: 10.3892/ol.2021.12932. Epub 2021 Jul 18. Oncol Lett. 2021. PMID: 34345296 Free PMC article.
References
- Olaussen K.A., Dunant A., Fouret P., Brambilla E., André F. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–991. - PubMed
- Tsang R.Y., Al-Fayea T., Au H.J. Cisplatin overdose: toxicities and management. Drug Saf. 2002;32:1109–1122. - PubMed
- Brabender J., Danenberg K.D., Metzger R., Schneider P.M., Park J. Epidermal growth factor receptor and HER2-neu mRNA expression in non-small cell lung cancer is correlated with survival. Clin Cancer Res. 2001;7:1850–1855. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous