YAP1 Regulates OCT4 Activity and SOX2 Expression to Facilitate Self-Renewal and Vascular Mimicry of Stem-Like Cells - PubMed (original) (raw)
YAP1 Regulates OCT4 Activity and SOX2 Expression to Facilitate Self-Renewal and Vascular Mimicry of Stem-Like Cells
Namrata Bora-Singhal et al. Stem Cells. 2015 Jun.
Abstract
Non-small cell lung cancer (NSCLC) is highly correlated with smoking and has very low survival rates. Multiple studies have shown that stem-like cells contribute to the genesis and progression of NSCLC. Our results show that the transcriptional coactivator yes-associated protein 1 (YAP1), which is the oncogenic component of the Hippo signaling pathway, is elevated in the stem-like cells from NSCLC and contributes to their self-renewal and ability to form angiogenic tubules. Inhibition of YAP1 by a small molecule or depletion of YAP1 by siRNAs suppressed self-renewal and vascular mimicry of stem-like cells. These effects of YAP1 were mediated through the embryonic stem cell transcription factor, Sox2. YAP1 could transcriptionally induce Sox2 through a physical interaction with Oct4; Sox2 induction occurred independent of TEAD2 transcription factor, which is the predominant mediator of YAP1 functions. The binding of Oct4 to YAP1 could be detected in cell lines as well as tumor tissues; the interaction was elevated in NSCLC samples compared to normal tissue as seen by proximity ligation assays. YAP1 bound to Oct4 through the WW domain, and a peptide corresponding to this region could disrupt the interaction. Delivery of the WW domain peptide to stem-like cells disrupted the interaction and abrogated Sox2 expression, self-renewal, and vascular mimicry. Depleting YAP1 reduced the expression of multiple epithelial-mesenchymal transition genes and prevented the growth and metastasis of tumor xenografts in mice; overexpression of Sox2 in YAP1 null cells rescued these functions. These results demonstrate a novel regulation of stem-like functions by YAP1, through the modulation of Sox2 expression.
Keywords: Non-small cell lung cancer; Self-renewal; Side-population cells; Transcriptional regulation; Vascular mimicry.
© 2015 AlphaMed Press.
Figures
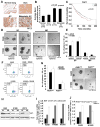
Figure 1. Higher expression of YAP1 in NSCLC CSCs
(A) Elevated YAP1 staining is seen in human lung adenocarcinoma and its metastatic sites as compared to the normal human lung tissue in TMA. SCC, Squamous cell carcinoma; AC, Adenocarcinoma. (B) Quantitation of IHC performed on the TMA shows a 3-fold increase in YAP1 expression in human lung adenocarcinoma tissue. (C) Significant correlation between higher levels of YAP1 and poor prognosis is observed by survival analysis of data from Director’s challenge set. (D) SP cells from A549 and H1650 cells show the ability to self-renew in serial sphere-formation assays in stem cell selective medium. (E) Quantitation of the average number of spheres formed from 1000 cells plated for serial sphere formation assay exhibit higher number of primary and secondary spheres in SP cells as compared to MP cells from A549 and H1650 cells. (F) Aldefluor staining of A549 and H1650 cells shows the presence of Aldhhigh population in both A549 and H1650 cells. Aldh activity inhibitor DEAB, is used to gate and separate the Aldhhigh cells. (G) Sphere formation assay and its quantitation show the ability of Aldhhigh population to form spheres in stem cell selective medium in both A549 and H1650 cells. The numbers of spheres are more in Aldhhigh population than the Aldhlow from both A549 and H1650 cells. (H) Western blot analysis on isolated SP and MP cells from NSCLC cell lines for YAP1 protein exhibit higher YAP1 protein levels in A549, H1650 and H1975 SP cells as compared to MP cells. β-Actin is used to show equal amounts of protein in the lanes. (I) qRT-PCR analysis of mRNA from SP and MP cells of A549, H1650 and H1975 cell lines reveal higher levels of YAP1 mRNA in SP cells. ABCG2 mRNA expression is used as positive control for SP cells (J) Elevated levels of YAP1 mRNA seen in sorted Aldhhigh cells compared to Aldhlow cells from A549 and H1650. The mRNA expression of Aldehyde dehydrogenase-1 (Aldh1) is used as a positive control.
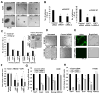
Figure 2. YAP1 silencing abrogates the self-renewal ability of CSCs
(A) Sphere formation assay with SP cells from A549 and H1650 transfected with two different siRNA against YAP1 show smaller spheres as compared to a non-target control siRNA (B) Average number of spheres generated from 1000 SP cells reveal fewer number of spheres in YAP1 siRNAs treated cells as compared to the control siRNA in both cell lines. (C) Quantitation of serial sphere formation assay show fewer spheres in SP cells isolated from A549 and H1650 cells transfected with YAP1 siRNAs as compare to control siRNA treatment. Bright field images of the spheres are presented here. (D) SP cells isolated from YAP1 siRNAs treated H1650 cells show abrogation of angiogenic tubule-like structure formation when grown on Matrigel in endothelial growth medium. (E) Loss of CD31 expression on angiogenic tubule-like structures in SP cells from YAP1 siRNA treated H1650 as visualized by immunofluorescence. (F) Real time PCR analysis of YAP1 siRNA treated H1650 cells show a decrease in CD31 mRNA expression as compared to control siRNA treated cells. (G–H) Real time PCR analysis of YAP1 siRNAs transfected A549 (G) and H1650 (H) cells respectively for YAP1, Sox2, Oct4, Nanog and ABCG2 genes show decrease in Sox2, Oct4, Nanog expression. The above data is expressed as mean ± SD of three independent experiments. * represents p < 0.05, ** represents p < 0.01.
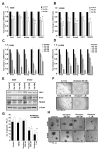
Figure 3. Visudyne inhibits self-renewal of CSCs
(A–B) Real time PCR (qRT-PCR) analysis of 1 μM Visudyne treated A549 (A) and H1650 (B) for 24 and 48 h respectively show decrease in stem cell factors like Sox2, Oct4, Nanog as well as YAP1 and TEAD2. (C–D) qRT-PCR analysis of expression of Sox2, Oct4, Nanog, YAP1 and TEAD2 upon treatment with various concentrations of Visudyne (0.5, 1, 2 and 5 μM) in A549 (C) and H1650 (D) cells for 48 hr. (E) Western blot analysis with 1 μM Visudyne treated A549 and H1650 cells exhibit a decrease in YAP1, Sox2 and TEAD2. β-Actin levels show equal amount of protein in all samples. (F) Visudyne treatment on SP cells sorted from H1650 cell line show decrease in angiogenic tubule-like structures when grown on Matrigel in endothelial cell growth medium. (G) Quantitation of spheres formed in a sphere formation assay with 1000 cells per treatment show fewer number of spheres with increasing concentration of Visudyne in SP cells from both A549 and H1650 cell lines. (H) Bright field images of spheres formed by A549 and H1650 SP cells exhibit decrease in self renewal ability of SP cells with Visudyne treatment. The above data is expressed as mean ± SD of three independent experiments. * represents p < 0.05, ** represents p < 0.01.
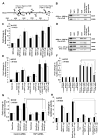
Figure 4. YAP1 regulates Sox2 gene expression through Oct4 transcription factor
(A) A schematic representation of Sox2 proximal core promoter and enhancer region 1 showing potential TEAD2 binding sites represented as clear ellipses and Oct4 binding sites as filled ellipses. The arrows represent the position of primers spanning the TEAD2 and Oct4 binding sites tested for ChIP assays. (B) ChIP assays conducted on asynchronously growing A549 and H1650 cells with the indicated antibodies show presence of YAP1 on SRR1 region. Acetylated Histone H3 (Lys9) was used as positive control and non-specific IgG was used as the negative control for immunoprecipitation. (C) Transient transfection experiments in A549 and H1650 cells with co-transfection with YAP1 and Oct4 expression vectors showed an additive effect on SRR1 enhancer-luc activity. Control lanes had the reporter with empty vector. (D) Presence of YAP1 on Sox2 core promoter and SRR1 enhancer region through Oct4 binding site is confirmed by ChIP assays conducted on asynchronously growing A549 and H1650 cells using the indicated antibodies. (E) Transient transfection assays in A549 and H1650 cells transfected with YAP1 or Oct4 expression vectors showed an increase in Sox2 core promoter-luc activity. An additive effect on Sox2 core promoter-luc activity is observed when YAP1 and Oct4 are co-expressed. (F) Sox2 core promoter-luc activity is reduced in both A549 and H1650 cells upon depletion of Oct4 using siRNA in the presence of exogenous YAP1; transfection of TEAD2, TEAD3 and TEAD4 siRNA had no significant effect. (G) Transient transfections in YAP1 depleted cells with siRNA treatment show reduction in Sox2 core promoter-luc activity with Oct4 overexpression as compared to non-target control siRNA treated A549 and H1650 cells. (H) Transient transfections of Sox2 core promoter-luc with mutated Oct4 binding (mutSox2-luc) site exhibit significantly reduced promoter activity even with increased exogenous YAP1 and Oct4 expression. The above data is expressed as mean ± SD of three independent experiments. * represents p < 0.05, ** represents p < 0.01.
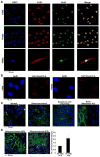
Figure 5. YAP1 directly associates with Oct4 transcription factor
(A) Immunofluorescence staining reveal YAP1 co-localizing with Oct4 in asynchronous A549 and H1650 NSCLC cell lines (top) and in human mesenchymal stem cells (hMSCs) (bottom). Representative confocal images of are presented here. DAPI is used to stain the nucleus. (B) Detection of YAP1-Oct4 protein-protein interaction using proximity ligation assay (PLA) in asynchronous A549 and H1650 cells. Multiple PLA foci are observed in the nucleus of the cells showing the association of YAP1 and Oct4 protein in both cell lines. (C) Proximity ligation assay performed on a human lung tissue microarray show strong association of YAP1 and Oct4 in lung tumor tissue as compared to the normal lung tissue. The red foci represent the PLA detection for YAP1 and Oct4 association. The TMA is also treated with anti-pan cytokeratin to detect the tumor areas in the tissue samples. DAPI is used to stain the nucleus. Representative confocal images captured for normal lung, adenocarcinoma, squamous cell carcinoma and metastatic adenocarcinoma to bone are shown here. (D) Proximity ligation assay on human lung tissue display a higher interaction of YAP1 and Oct4 proteins in poorly differentiated as compared to well differentiated lung adenocarcinoma. The quantitation of fold change in number of foci per cell shows a 2-fold increase in the signal from poorly differentiated lung adenocarcinomas. Adenocarcinoma well differentiated (WD), Adenocarcinoma poorly differentiated (PD).
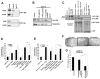
Figure 6. YAP1 WW domain interacts with Oct4 transcription factor
(A) YAP1-Oct4 binding is detected by immunoprecipitation with an Oct4 antibody followed by western blot analysis with YAP1 in asynchronous A549 and H1650 cells. Normal IgG was used as a control for the IP reaction, and western blot for E2F was a negative control for Oct4 binding. (B) YAP1-Oct4 interaction is disrupted by a WW domain peptide. The presence of Oct4 in YAP1 immunoprecipitation was abolished by the WW domain peptide, but not a scrambled peptide, as seen by western blotting; binding of TEAD2 to YAP1 was not affected. Normal IgG is used as a control for the IP reaction. (C) An in vitro binding assay using 293T cell lysate overexpressing wild type YAP1 (YAP1 WT OE) or WW mutant YAP1 (YAP1 WW mutant OE) and GST-Oct4 showing that mutation in the WW domain of YAP1 abrogates its binding to Oct4. (D) Expression of YAP1 WW domain mutant lacks the ability to induce the Sox2 core promoter-luc activity alone or in the presence of Oct4 exogenous expression as shown in a luciferase assay. The increased Sox2 core promoter-luc activity is seen when YAP1 WT construct was used alone or in combination with Oct4. 1 μg of DNA is used for all the plasmid constructs in this assay (E) WW domain peptide conjugated to penetratin (WW peptide conjugate) inhibited the YAP1 mediated induction of Sox2 core promoter-luc activity, but a scrambled peptide conjugated to penetratin (Scrambled peptide conjugate) did not. (F) WW peptide conjugate, but not a scrambled peptide conjugate, abrogates angiogenic tubule formation by H1650 SP cells (G) SP cells from A549 and H1650 cell lines form less number of spheres when with WW peptide conjugate, but not scrambled peptide conjugate. The above data is represented as mean ± SD of three independent experiments. * represents p < 0.05, ** represents p < 0.01.
Figure 7. Sox2 expression rescues loss of stem-like and tumor growing features of YAP1 null cells
(A–B) qRT-PCR analysis of A549 (A) and H1650 (B) cells treated with YAP1 WW peptide conjugated to penetratin for 48 h show a change in EMT related genes as compared to cells treated with Scrambled peptide penetratin conjugate. (C) Real time PCR analysis of two clones of YAP1 null H1650-luc cells (clones C1 and C2) show a reduction in YAP1, Sox2 and Oct4 mRNA expression as compared to shControl H1650-luc cells. Also, a significant change in mRNA of genes associated with epithelial and mesenchymal features is observed in both YAP1 null clones (C1 and C2). (D) Western blot analysis reveal decrease in YAP1 and Sox2 protein expression in YAP1 null H1650-luc cells as compared to shControl H1650-luc cells. A marked reduction in the levels of mesenchymal proteins that change during EMT is also seen with YAP1 knock down in H1650-luc cells. (E) YAP1 null H1650-luc cells (clone C1 and C2) show reduced invasive properties as seen in Boyden chamber assay in comparison with shControl H1650 cells. The representative bright field images of the cells on the membrane are shown here. (F) Tumor growth monitored in mice injected with shYAP1 H1650-luc cells showed significantly lower tumor growth as compared to mice injected with shControl cells. (G) Western blot analysis shows the overexpression of Sox2 protein in shControl and shYAP1 H1650-luc cells stably transfected with a Sox2 expression vector. (H) Depletion of Sox2 or YAP1 reduced the growth of orthotopically implanted tumors as compared to shControl H1650-luc cells. Stable over expression of Sox2 rescued the tumor growing abilities of YAP1 knock down H1650-luc cells. (I–J) SP cells from H1650-luc cells showed significantly reduced self-renewal when Sox2 or YAP1 was depleted, as seen in a sphere formation assay; sphere forming capacity of YAP1 knock depleted was rescued by stable overexpression of Sox2. The above data represents the mean ± SD, *, p < 0.05, **, p < 0.01 (K) Schematic representation of proposed mechanism of YAP1 mediated regulation of stemness and in NSCLC SP cells. TBD, Tead binding domain; WW, WW domain; PPxY, PPxY motif.
Similar articles
- Regulation of Sox2 and stemness by nicotine and electronic-cigarettes in non-small cell lung cancer.
Schaal CM, Bora-Singhal N, Kumar DM, Chellappan SP. Schaal CM, et al. Mol Cancer. 2018 Oct 15;17(1):149. doi: 10.1186/s12943-018-0901-2. Mol Cancer. 2018. PMID: 30322398 Free PMC article. - Yap1 is dispensable for self-renewal but required for proper differentiation of mouse embryonic stem (ES) cells.
Chung H, Lee BK, Uprety N, Shen W, Lee J, Kim J. Chung H, et al. EMBO Rep. 2016 Apr;17(4):519-29. doi: 10.15252/embr.201540933. Epub 2016 Feb 25. EMBO Rep. 2016. PMID: 26917425 Free PMC article. - EGFR/Src/Akt signaling modulates Sox2 expression and self-renewal of stem-like side-population cells in non-small cell lung cancer.
Singh S, Trevino J, Bora-Singhal N, Coppola D, Haura E, Altiok S, Chellappan SP. Singh S, et al. Mol Cancer. 2012 Sep 25;11:73. doi: 10.1186/1476-4598-11-73. Mol Cancer. 2012. PMID: 23009336 Free PMC article. - Yin Yang 1 is associated with cancer stem cell transcription factors (SOX2, OCT4, BMI1) and clinical implication.
Kaufhold S, Garbán H, Bonavida B. Kaufhold S, et al. J Exp Clin Cancer Res. 2016 May 25;35:84. doi: 10.1186/s13046-016-0359-2. J Exp Clin Cancer Res. 2016. PMID: 27225481 Free PMC article. Review. - Sox2/Oct4: A delicately balanced partnership in pluripotent stem cells and embryogenesis.
Rizzino A, Wuebben EL. Rizzino A, et al. Biochim Biophys Acta. 2016 Jun;1859(6):780-91. doi: 10.1016/j.bbagrm.2016.03.006. Epub 2016 Mar 23. Biochim Biophys Acta. 2016. PMID: 26992828 Review.
Cited by
- Modulating versatile pathways using a cleavable PEG shell and EGFR-targeted nanoparticles to deliver CRISPR-Cas9 and docetaxel for triple-negative breast cancer inhibition.
Lo YL, Hong CJ, Wang CS, Yang CP. Lo YL, et al. Arch Pharm Res. 2024 Nov;47(10-11):829-853. doi: 10.1007/s12272-024-01514-0. Epub 2024 Nov 1. Arch Pharm Res. 2024. PMID: 39482441 Free PMC article. - ALK1 Signaling in Human Cardiac Progenitor Cells Promotes a Pro-angiogenic Secretome.
Moore M, Ryzhov S, Sawyer DB, Gartner C, Vary CPH. Moore M, et al. J Cell Signal. 2024;5(3):122-142. doi: 10.33696/signaling.5.119. J Cell Signal. 2024. PMID: 39430425 Free PMC article. - Defective N-glycosylation of IL6 induces metastasis and tyrosine kinase inhibitor resistance in lung cancer.
Hung CH, Wu SY, Yao CD, Yeh HH, Lin CC, Chu CY, Huang TY, Shen MR, Lin CH, Su WC. Hung CH, et al. Nat Commun. 2024 Sep 9;15(1):7885. doi: 10.1038/s41467-024-51831-7. Nat Commun. 2024. PMID: 39251588 Free PMC article. - Competing endogenous RNAs regulatory crosstalk networks: The messages from the RNA world to signaling pathways directing cancer stem cell development.
Aria H, Azizi M, Nazem S, Mansoori B, Darbeheshti F, Niazmand A, Daraei A, Mansoori Y. Aria H, et al. Heliyon. 2024 Jul 26;10(15):e35208. doi: 10.1016/j.heliyon.2024.e35208. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39170516 Free PMC article. Review. - A highly sensitive reporter system to monitor endogenous YAP1/TAZ activity and its application in various human cells.
Hikasa H, Kawahara K, Inui M, Yasuki Y, Yamashita K, Otsubo K, Kitajima S, Nishio M, Arima K, Endo M, Taira M, Suzuki A. Hikasa H, et al. Cancer Sci. 2024 Oct;115(10):3370-3383. doi: 10.1111/cas.16316. Epub 2024 Aug 18. Cancer Sci. 2024. PMID: 39155534 Free PMC article.
References
- Seve P, Dumontet C. Chemoresistance in non-small cell lung cancer. Curr Med Chem Anticancer Agents. 2005;5:73–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA139612/CA/NCI NIH HHS/United States
- R01 CA139612/CA/NCI NIH HHS/United States
- R25 CA174664/CA/NCI NIH HHS/United States
- P30 CA076292/CA/NCI NIH HHS/United States
- R01 CA127725/CA/NCI NIH HHS/United States
- CA127725/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials