Successful Interferon-Free Therapy of Chronic Hepatitis C Virus Infection Normalizes Natural Killer Cell Function - PubMed (original) (raw)
Successful Interferon-Free Therapy of Chronic Hepatitis C Virus Infection Normalizes Natural Killer Cell Function
Elisavet Serti et al. Gastroenterology. 2015 Jul.
Abstract
Background & aims: Chronic hepatitis C virus infection activates an intrahepatic immune response, leading to increased expression of interferon (IFN)-stimulated genes and activation of natural killer (NK) cells-the most prevalent innate immune cell in the liver. We investigated whether the elimination of hepatitis C virus with direct-acting antiviral normalizes expression of IFN-stimulated genes and NK cell function.
Methods: We used multicolor flow cytometry to analyze NK cells from the liver and blood of 13 HCV-infected patients who did not respond to treatment with pegylated interferon and ribavirin. Samples were collected before and during IFN-free treatment with daclatasvir and asunaprevir and compared with samples from the blood of 13 healthy individuals (controls). Serum levels of chemokine C-X-C motif ligand (CXCL) 10 or CXCL11 were measured by enzyme-linked immunosorbent assay.
Results: Before treatment, all patients had increased levels of CXCL10 or CXCL11 and a different NK cell phenotype from controls, characterized by increased expression of HLA-DR, NKp46, NKG2A, CD85j, signal transducer and activator of transcription 1 (STAT1), phosphorylated STAT1, and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). NK cells from patients also had increased degranulation and decreased production of IFNγ and tumor necrosis factor α compared with NK cells from controls. Nine patients had an end-of-treatment response (undetectable virus) and 4 had virologic breakthrough between weeks 4 and 12 of therapy. A rapid decrease in viremia and level of inflammatory cytokines in all patients was associated with decreased activation of intrahepatic and blood NK cells; it was followed by restoration of a normal NK cell phenotype and function by week 8 in patients with undetectable viremia. This normalized NK cell phenotype was maintained until week 24 (end of treatment).
Conclusions: Direct-acting antiviral-mediated clearance of HCV is associated with loss of intrahepatic immune activation by IFNα, which is indicated by decreased levels of CXCL10 and CXCL11 and normalization of NK cell phenotype and function.
Keywords: ISG; Immune Regulation; NS3 Inhibitor; NS5A Inhibitor.
Copyright © 2015 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures
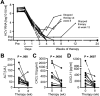
Figure 1. Serum HCV RNA and liver inflammation rapidly decrease with DCV/ASV therapy
(A) Serum HCV RNA levels of patients who responded to therapy (n=9, filled circles) or had a viral breakthrough and subsequently stopped therapy (n=4, filled squares). A response to DCV/ASV therapy was defined as undetectable viremia at EOT (week 24). ‘Pre’ indicates the time point of the pre-treatment liver biopsy (1 - 4 weeks prior to therapy). L.l.o.q., lower limit of quantitation; ‘t.n.d.’, target not detected. (B-D) Serum ALT (B), CXCL10 (C) and CXCL11 (D) levels of all patients
(n=13)
at week 0 and at week 8 of therapy. Filled circles, EOT responders; filled triangles, patients with virological breakthrough. The patient with increased ALT value is patient 12 in Suppl. Table 1.
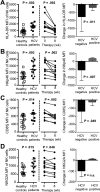
Figure 2. NK cell activation decreases within 8 weeks of successful DCV/ASV therapy
(A-D) Expression of the activation markers HLA-DR (A), NKp46 (B) and the inhibitory receptors CD85j (C) and NKG2A (D) on peripheral blood NK cells of chronic HCV patients prior to treatment (n=13) compared to NK cells of healthy controls (white squares, left graphs). Change in the expression of these NK cell markers in 10 patients who had undetectable viremia (middle graph) compared to 3 patients who were viremic at week 8 (right graphs). Statistical analysis: non-parametric paired Wilcoxon-signed-rank test or unpaired Mann-Whitney test. Median and IQR are shown. Filled circles, EOT responders; filled triangle, patient with virological breakthrough after week 8.
Figure 3. Increased NK cell cytotoxicity normalizes within 8 weeks of successful DCV/ASV therapy
(A-E) Frequency CD107a+ cells (A), CD107a expression level (B), frequency of TRAIL+ cells (C), TRAIL expression level (D) and STAT1 expression level (E) in the CD56dim NK cell population of HCV-infected patients prior to treatment (n=13) compared to NK cells of healthy controls (white squares, left graphs). Change in these parameters in 10 patients who had undetectable viremia (middle graphs) compared to 3 patients who were viremic at week 8 (right graphs). (F) Frequency of pSTAT1+ cells and pSTAT1 expression level in the CD56dim NK cell population of HCV-infected patients prior to treatment (n=13) compared to NK cells of healthy controls (left graphs). Change in these parameters in 10 patients who had undetectable viremia at week 8 (right graphs). Statistical analysis: non-parametric paired Wilcoxon-signed-rank test or unpaired Mann-Whitney test. Median and IQR are shown. Filled circles, EOT responders; filled triangle, patient with virological breakthrough after week 8. MFI, mean fluorescence intensity.
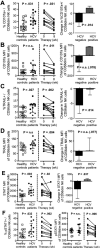
Figure 4. Decreased IFNγ and TNFα production by NK cells is restored within 8 weeks of successful DCV/ASV therapy
(A) Frequency of IFNγ+ cells, (B) IFNγ expression level, and frequency of (C) TNFα+ and (D) IFNγ+TNFα+ in the CD56bright NK cell population of chronic HCV patients prior to treatment (n=13) compared to NK cells of healthy controls (white squares, left graphs). Change in these parameters in 10 patients who had undetectable viremia at week 8 (right graphs). Statistical analysis: non-parametric paired Wilcoxon-signed-rank test or unpaired Mann-Whitney test. Median and IQR are shown. Filled circles, EOT responders; filled triangle, patient with virological breakthrough after week 8. MFI, mean fluorescence intensity.
Figure 5. Activation and TRAIL expression of intrahepatic CD56dim NK cells decrease within 2-4 weeks of DCV/ASV therapy in parallel to decreasing viremia and liver inflammation
(A-B) Comparison of frequency and expression level of CD69+ (A) and NKp46+ (B) cells in peripheral and intrahepatic CD56dim NK cell populations of chronically HCV-infected patients prior to therapy. (C-D) Frequency and expression level of TRAIL+ (C) and NKp46+ (D) CD56dim NK cells in paired liver biopsies prior to (‘Pre’) and, depending on randomization, either at week 2 or at week 4 of therapy. Horizontal marks identify week 2 biopsies. Statistical analysis: non-parametric paired Wilcoxon-signed-rank test. Filled circles, EOT responders; filled triangle, patient with virological breakthrough after week 8. MFI, mean fluorescence intensity.
Figure 6. NK cell functions normalize in a sequential manner during DCV/ASV therapy
Changes in HLA-DR expression in all CD56+ NK cells (A) and in the frequency of IFNγ+ CD56bright (B) and CD107+ CD56dim (C) NK cells during the first 24 hours (left graphs) and the first 2 weeks (right graphs) of therapy.
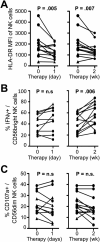
Figure 7. Normalized NK cell phenotype and function are maintained in EOT responders to DCV/ASV therapy
(A-B) Expression of the activation marker HLA-DR, the activating receptor NKp46, the inhibitory receptor CD85j (A) and the chemokine receptor CXCR3 (B) on peripheral blood CD56+ NK cells of EOT responders at weeks 0 and 24 of therapy. (C-D) Frequency of CD107a+ and TRAIL+ cells (C) and frequency of pSTAT1+ cells and STAT1 expression level (D) in the CD56dim NK cell population of EOT responders at weeks 0 and 24 of therapy. (D) in the CD56dim NK cell population of EOT responders at weeks 0 and 24 of therapy. (E) Frequency of IFNγ+, TNFα+ and IFNγ+TNFα+ cells and IFNγ expression level in the peripheral blood CD56bright NK cell population of EOT responders at weeks 0 and 24 of therapy. Statistical analysis: non-parametric paired Wilcoxon-signed-rank test.
Comment in
- Direct-Acting Antivirals Cure Innate Immunity in Chronic Hepatitis C.
Mondelli MU. Mondelli MU. Gastroenterology. 2015 Jul;149(1):25-8. doi: 10.1053/j.gastro.2015.05.026. Epub 2015 May 27. Gastroenterology. 2015. PMID: 26021236 No abstract available.
Similar articles
- Rapid decrease in hepatitis C viremia by direct acting antivirals improves the natural killer cell response to IFNα.
Serti E, Park H, Keane M, O'Keefe AC, Rivera E, Liang TJ, Ghany M, Rehermann B. Serti E, et al. Gut. 2017 Apr;66(4):724-735. doi: 10.1136/gutjnl-2015-310033. Epub 2016 Jan 4. Gut. 2017. PMID: 26733671 Free PMC article. - Cirrhosis Hampers Early and Rapid Normalization of Natural Killer Cell Phenotype and Function in Hepatitis C Patients Undergoing Interferon-Free Therapy.
Perpiñán E, Pérez-Del-Pulgar S, Londoño MC, Mariño Z, Bartres C, González P, García-López M, Pose E, Lens S, Maini MK, Forns X, Koutsoudakis G. Perpiñán E, et al. Front Immunol. 2020 Feb 25;11:129. doi: 10.3389/fimmu.2020.00129. eCollection 2020. Front Immunol. 2020. PMID: 32161581 Free PMC article. - Interferon-alpha-induced TRAIL on natural killer cells is associated with control of hepatitis C virus infection.
Stegmann KA, Björkström NK, Veber H, Ciesek S, Riese P, Wiegand J, Hadem J, Suneetha PV, Jaroszewicz J, Wang C, Schlaphoff V, Fytili P, Cornberg M, Manns MP, Geffers R, Pietschmann T, Guzmán CA, Ljunggren HG, Wedemeyer H. Stegmann KA, et al. Gastroenterology. 2010 May;138(5):1885-97. doi: 10.1053/j.gastro.2010.01.051. Epub 2010 Feb 2. Gastroenterology. 2010. PMID: 20334827 - Natural killer cells in hepatitis C: Current progress.
Yoon JC, Yang CM, Song Y, Lee JM. Yoon JC, et al. World J Gastroenterol. 2016 Jan 28;22(4):1449-60. doi: 10.3748/wjg.v22.i4.1449. World J Gastroenterol. 2016. PMID: 26819513 Free PMC article. Review. - Immunomodulation of CXCL10 Secretion by Hepatitis C Virus: Could CXCL10 Be a Prognostic Marker of Chronic Hepatitis C?
Ferrari SM, Fallahi P, Ruffilli I, Elia G, Ragusa F, Paparo SR, Patrizio A, Mazzi V, Colaci M, Giuggioli D, Ferri C, Antonelli A. Ferrari SM, et al. J Immunol Res. 2019 Aug 8;2019:5878960. doi: 10.1155/2019/5878960. eCollection 2019. J Immunol Res. 2019. PMID: 31485460 Free PMC article. Review.
Cited by
- Hepatitis C Virus Relapse After Ultrashort Direct-Acting Antiviral Therapy Associates With Expression of Genes Involved With Natural Killer-Cell and CD8+ T-Cell Function.
Orr C, Masur H, Kottilil S, Meissner EG. Orr C, et al. Open Forum Infect Dis. 2021 Mar 13;8(4):ofab118. doi: 10.1093/ofid/ofab118. eCollection 2021 Apr. Open Forum Infect Dis. 2021. PMID: 33959672 Free PMC article. - Lymphocyte Landscape after Chronic Hepatitis C Virus (HCV) Cure: The New Normal.
Ghosh A, Romani S, Kottilil S, Poonia B. Ghosh A, et al. Int J Mol Sci. 2020 Oct 10;21(20):7473. doi: 10.3390/ijms21207473. Int J Mol Sci. 2020. PMID: 33050486 Free PMC article. Review. - Liver cancer: Effect of HCV clearance with direct-acting antiviral agents on HCC.
Llovet JM, Villanueva A. Llovet JM, et al. Nat Rev Gastroenterol Hepatol. 2016 Oct;13(10):561-2. doi: 10.1038/nrgastro.2016.140. Epub 2016 Sep 1. Nat Rev Gastroenterol Hepatol. 2016. PMID: 27580683 No abstract available. - Two Cases of Hepatocellular Carcinoma Occurring Immediately after Direct-acting Antiviral Agents against Hepatitis C Virus.
Nishijima N, Nasu A, Kimura T, Osaki Y. Nishijima N, et al. Intern Med. 2019 Jan 15;58(2):225-231. doi: 10.2169/internalmedicine.0712-17. Epub 2018 Aug 24. Intern Med. 2019. PMID: 30146562 Free PMC article. - Risk of Hepatocellular Cancer Recurrence in Hepatitis C Virus+ Patients Treated with Direct-Acting Antiviral Agents.
Zou WY, Choi K, Kramer JR, Yu X, Cao Y, El-Serag HB, Kanwal F. Zou WY, et al. Dig Dis Sci. 2019 Nov;64(11):3328-3336. doi: 10.1007/s10620-019-05641-3. Epub 2019 Apr 30. Dig Dis Sci. 2019. PMID: 31041639 Free PMC article.
References
- Askarieh G, Alsio A, Pugnale P, et al. Systemic and intrahepatic interferon-gamma-inducible protein 10 kDa predicts the first-phase decline in hepatitis C Virus RNA and overall viral response to therapy in chronic hepatitis C. Hepatology. 2010;51:1523–1530. - PubMed
- Doherty DG, O'Farrelly C. Innate and adaptive lymphoid cells in the human liver. Immunological Rev. 2000;174:5–20. - PubMed
- Miyagi T, Takehara T, Nishio K, et al. Altered interferon-alpha-signaling in natural killer cells from patients with chronic hepatitis C virus infection. J Hepatol. 2010;53:424–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous