Rapid, optimized interactomic screening - PubMed (original) (raw)
doi: 10.1038/nmeth.3395. Epub 2015 May 4.
Michal Domanski 2, Loren E Hough 1, Asha A Oroskar 3, Anil R Oroskar 3, Sarah Keegan 4, David J Dilworth 5, Kelly R Molloy 6, Vadim Sherman 7, John D Aitchison 5, David Fenyö 4, Brian T Chait 6, Torben Heick Jensen 8, Michael P Rout 1, John LaCava 1
Affiliations
- PMID: 25938370
- PMCID: PMC4449307
- DOI: 10.1038/nmeth.3395
Rapid, optimized interactomic screening
Zhanna Hakhverdyan et al. Nat Methods. 2015 Jun.
Abstract
We must reliably map the interactomes of cellular macromolecular complexes in order to fully explore and understand biological systems. However, there are no methods to accurately predict how to capture a given macromolecular complex with its physiological binding partners. Here, we present a screening method that comprehensively explores the parameters affecting the stability of interactions in affinity-captured complexes, enabling the discovery of physiological binding partners in unparalleled detail. We have implemented this screen on several macromolecular complexes from a variety of organisms, revealing novel profiles for even well-studied proteins. Our approach is robust, economical and automatable, providing inroads to the rigorous, systematic dissection of cellular interactomes.
Conflict of interest statement
Competing interests
A.A.O., A.R.O., M.P.R., and J.L. are inventors on a US patent application encompassing the filter work described in this manuscript.
Figures
Figure 1
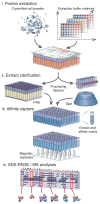
Schematic representation of the parallelized affinity capture procedure. (i) cells expressing a tagged protein of interest are mechanically disrupted at cryogenic temperature to produce a micron-scale powder and precise aliquots of the frozen powder are deposited into the wells of a multi-well plate using a dedicated manifold. A diverse set of extraction solvents are rapidly added in parallel and complete re-suspension with concomitant extraction of the powder is ensured using immediate brief mechanical agitation and/or low-power sonication; (ii) rapid removal of insoluble material is achieved by either centrifugation or using a novel deep-bed filtration device (this study); (iii) affinity capture is then performed on the clarified extract using magnetic beads coupled with an affinity reagent for the tag used, and a multipronged magnet separator in register with the multiwell plate, allowing subsequent washing steps; (iv) elution of the complexes is followed by SDS-PAGE, Coomassie blue staining, and (as desired) MS analysis. The resulting copurification profiles are catalogued and cross-compared to infer interactomes and determine preparative conditions appropriate for further biochemical and analytical means.
Figure 2
Dispensing manifold and filtration device. (a) Schematic representations of the manifold used to dispense a calibrated amount of frozen cell powder into a 96-well plate. A set of adapters and volume displacing prongs are used to deliver the required amount of cell material. (b) Schematic representation of the filtration device used to clarify crude yeast cell extracts. Each well contains a composite filter comprised of multiple filtration elements, including: a coarse pre-filter that retards the flow of highly aggregated material; a diatomaceous earth depth filter that permits the passage of soluble material, and traps the insoluble debris that can clog submicron filters; and a 0.2 micron membrane filter, which provides a uniform final clarification. (c) Coomassie blue stained SDS-PAGE analysis of Nup53p-SpA affinity capture (100mg cell material resuspended with 600uL extraction solvent: 40 mM TRIS-Cl, pH 8, 250 mM trisodium citrate, 150 mM NaCl, 1% v/v Triton X-100) comparing extract clarification by centrifugation at 14k rpm for 10 min (“Centrifuged”) and filtration at 3.5k rpm for 5 min (“Filtered”), MW – molecular weight standard. Duplicate experiments produced identical results (not shown). Proteins labeled in accordance with Figure 4a. (d) Pictures of the actual devices from left to right: adjustable volume dispensing manifold (as in a), shown bottom up; dispensing manifold with 96-well deep-well plate atop, cell material transfer is achieved upon inversion of this assembly; a 96-well filtration device (as inb) atop a 96-well, deep well collection plate.
Figure 3
Extraction condition design and copurification pattern analysis. (a) Flow-chart representation of mixtures of components in select extraction solvent formulations. The main components are a pH buffer, 1 or 2 salts, an additive and a detergent. Some examples of useful formulations discovered through screening are indicated (refer to Fig. 5 to view the associated copurification patterns, indicated by Roman numerals). (b) Comparison of SDS-PAGE (“Gel”) and LC-MS/MS (“MS”) clustering analysis of Nup1p-SpA 96-well purification. For a visual comparison the MS data is represented as a pseudo-gel, where each band corresponds to a protein above a certain intensity threshold (see Methods). Known Nup1p interacting proteins are indicated with blue bands, the rest are labeled black. Co-clustering conditions with identical or highly similar components producing distinct copurification profiles are highlighted in blue (low ammonium acetate or low potassium acetate), orange (high sodium citrate/high ammonium acetate with Triton X-100) and green (sodium citrate/potassium acetate with CHAPS) boxes. See Supplementary Figure 3 for lane labels.
Figure 4
NPC purification – from single proteins to macromolecular assemblies. (a) A representative SDS-PAGE image and MS analysis of affinity capture of 3 nucleoporins – Nup1p, Nup53p and Pom152p. Composition of affinity isolation solvents: i – 50 mM trisodium citrate, 300 mM NaCl, 10 mM Tween 20, 2 mM EDTA, 40 mM TRIS, pH 8; ii – 250 mM trisodium citrate, 10 mM Brij58, 0.3 mM Sarkosyl, 40 mM TRIS, pH 8; iii - 1.5 M ammonium acetate, 15 mM Triton X-100. The protein bands identified by MS are marked on the gel. The table below contains the list of identified proteins. Affinity tagged nups are labeled red, the remaining NPC constituents are black and non-NPC proteins are gray. Each protein identified by MS is marked by a dot under the corresponding lane. The brackets indicate comigrating proteins identified in a single band. (b) Section through the density map of the NPC with one spoke enlarged and minimum Chimera representations of NPC subcomplexes in (a) for 1 spoke.
Figure 5
Affinity capture strategy implementation on different protein complexes, affinity tags and model organisms. Affinity capture profiles: (a) S. cerevisiae; (b) E. coli; (c) H. sapiens. Representative SDS-PAGE profiles are shown. The cell schematics indicate the localization of the tagged proteins; the different tagged proteins screened are indicated in black; each lane corresponds to a different purification and is assigned an arbitrary Roman numeral (see Supplementary Table 1 for extraction conditions, except when specified). Some of the newly identified putative interactors were subjected to affinity capture (labeled brown) and the resulting profiles are indicated by arced arrows originating from the profiles in which they were identified. Copurifying protein bands identified by MS are marked next to each profile (see Supplementary Data). Protein names marked in blue are previously characterized physical interactors, those in red are select novel physical interactors or proteins of interest discussed in the main text, and those in grey are contaminants or proteins of indeterminate specificity based on their high frequency of copurification, or as determined by I-DIRT analysis (Fig. 6a). The bands labeled with an “*” indicate heavy and light chains of the antibody used for affinity capture. Ent2p and End3p extraction solvent – 40mM TRIS-Cl, pH 8, 150 mM NaCl, 250 mM trisodium citrate, 10 mM deoxy-BigCHAP, Tcb1p, Tcb2p, Tcb3p, and Dpm1p extraction solvent - 40 mM TRIS-Cl, pH 8, 150 mM NaCl, 50 mM trisodium citrate, 5 mM CHAPS.
Figure 6
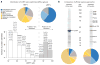
An in-depth analysis of Rtn1p affinity capture. (a) Frequency distribution of I-DIRT ratios – light protein intensity/total protein intensity, normalized to 100% – from Rtn1p-GFP affinity capture experiment (extraction condition as in Fig. 5a, Rtn1p-GFP). Putative in vivo interactors are represented with shaded bars (≥85%), likely post-extraction associations – with open bars (<85%). Representative proteins are shown above each bar, proteins labeled in Figure 5a (Rtn1p-GPF) and known interactors of Rtn1p are bolded. The proteins with <85% and ≥85% I-DIRT ratio were separately analyzed for the subcellular localization and molecular function. The corresponding pie charts are plotted above the I-DIRT ratio distribution (see Methods for details). (b) SDS-PAGE and MS comparison of standard and optimized affinity capture. 4g of Rtn1p-TAP powder was processed essentially as previously described using Triton X-100 as a detergent in a 2-step affinity capture experiment and 0.4g of Rtn1p-TAP was processed in a 1-step affinity capture experiment using conditions revealed in the present study (Fig. 5a, Rtn1p-GFP). Half of the elution was analyzed by SDS-PAGE followed by Coomassie staining and the other half was analyzed by LC-MS/MS. The distribution of subcellular localizations and molecular functions was analyzed as in (a) and is plotted bellow the corresponding gel lanes.
Figure 7
Robotic implementation. SDS-PAGE analysis of one Nup53p-SpA purification screen performed using a Hamilton STAR liquid handling workstation, testing 50 – 300 mM trisodium citrate (40mM TRIS-Cl, pH 8, 1% v/v Triton X-100 are common to all lanes). The purifications form bracketed lanes were manually repeated and analyzed by MALDI-TOF-MS (Supplementary Data). Three distinct profiles are observed: copurification with Nup170p and Kap121p (i); dimer with Nup170p (ii); and a larger subcomplex of the NPC: Nup192p, Nup188p, Nup170p, Nup116p, Nup120p, Nsp1p and Nic96p (iii).
Similar articles
- Analyzing protein-protein interactions in the post-interactomic era. Are we ready for the endgame?
Johnsson N. Johnsson N. Biochem Biophys Res Commun. 2014 Mar 21;445(4):739-45. doi: 10.1016/j.bbrc.2014.02.023. Epub 2014 Feb 15. Biochem Biophys Res Commun. 2014. PMID: 24548408 Review. - A high-throughput approach for measuring temporal changes in the interactome.
Kristensen AR, Gsponer J, Foster LJ. Kristensen AR, et al. Nat Methods. 2012 Sep;9(9):907-9. doi: 10.1038/nmeth.2131. Epub 2012 Aug 5. Nat Methods. 2012. PMID: 22863883 Free PMC article. - Novel approaches to map protein interactions.
Figeys D. Figeys D. Curr Opin Biotechnol. 2003 Feb;14(1):119-25. doi: 10.1016/s0958-1669(02)00005-8. Curr Opin Biotechnol. 2003. PMID: 12566011 Review. - Quantitative Tagless Copurification: A Method to Validate and Identify Protein-Protein Interactions.
Shatsky M, Dong M, Liu H, Yang LL, Choi M, Singer ME, Geller JT, Fisher SJ, Hall SC, Hazen TC, Brenner SE, Butland G, Jin J, Witkowska HE, Chandonia JM, Biggin MD. Shatsky M, et al. Mol Cell Proteomics. 2016 Jun;15(6):2186-202. doi: 10.1074/mcp.M115.057117. Epub 2016 Apr 20. Mol Cell Proteomics. 2016. PMID: 27099342 Free PMC article. - Identification of mammalian protein complexes by lentiviral-based affinity purification and mass spectrometry.
Ni Z, Olsen JB, Emili A, Greenblatt JF. Ni Z, et al. Methods Mol Biol. 2011;781:31-45. doi: 10.1007/978-1-61779-276-2_2. Methods Mol Biol. 2011. PMID: 21877275
Cited by
- Affinity Proteomic Analysis of the Human Exosome and Its Cofactor Complexes.
Winczura K, Domanski M, LaCava J. Winczura K, et al. Methods Mol Biol. 2020;2062:291-325. doi: 10.1007/978-1-4939-9822-7_15. Methods Mol Biol. 2020. PMID: 31768983 - Integrative structure and functional anatomy of a nuclear pore complex.
Kim SJ, Fernandez-Martinez J, Nudelman I, Shi Y, Zhang W, Raveh B, Herricks T, Slaughter BD, Hogan JA, Upla P, Chemmama IE, Pellarin R, Echeverria I, Shivaraju M, Chaudhury AS, Wang J, Williams R, Unruh JR, Greenberg CH, Jacobs EY, Yu Z, de la Cruz MJ, Mironska R, Stokes DL, Aitchison JD, Jarrold MF, Gerton JL, Ludtke SJ, Akey CW, Chait BT, Sali A, Rout MP. Kim SJ, et al. Nature. 2018 Mar 22;555(7697):475-482. doi: 10.1038/nature26003. Epub 2018 Mar 14. Nature. 2018. PMID: 29539637 Free PMC article. - Dissecting the Structural Dynamics of the Nuclear Pore Complex.
Hakhverdyan Z, Molloy KR, Keegan S, Herricks T, Lepore DM, Munson M, Subbotin RI, Fenyö D, Aitchison JD, Fernandez-Martinez J, Chait BT, Rout MP. Hakhverdyan Z, et al. Mol Cell. 2021 Jan 7;81(1):153-165.e7. doi: 10.1016/j.molcel.2020.11.032. Epub 2020 Dec 16. Mol Cell. 2021. PMID: 33333016 Free PMC article. - Purification and analysis of endogenous human RNA exosome complexes.
Domanski M, Upla P, Rice WJ, Molloy KR, Ketaren NE, Stokes DL, Jensen TH, Rout MP, LaCava J. Domanski M, et al. RNA. 2016 Sep;22(9):1467-75. doi: 10.1261/rna.057760.116. Epub 2016 Jul 11. RNA. 2016. PMID: 27402899 Free PMC article. - Affinity Isolation of Endogenous Saccharomyces Cerevisiae Nuclear Pore Complexes.
Nudelman I, Fernandez-Martinez J, Rout MP. Nudelman I, et al. Methods Mol Biol. 2022;2502:3-34. doi: 10.1007/978-1-0716-2337-4_1. Methods Mol Biol. 2022. PMID: 35412228 Free PMC article.
References
- Brockhurst MA, Colegrave N, Rozen DE. Next-generation sequencing as a tool to study microbial evolution. Mol Ecol. 2011;20:972–980. - PubMed
- Ross JS, Cronin M. Whole cancer genome sequencing by next-generation methods. Am J Clin Pathol. 2011;136:527–539. - PubMed
- Charbonnier S, Gallego O, Gavin AC. The social network of a cell: recent advances in interactome mapping. Biotechnol Annu Rev. 2008;14:1–28. - PubMed
- Collins MO, Choudhary JS. Mapping multiprotein complexes by affinity purification and mass spectrometry. Curr Opin Biotechnol. 2008;19:324–330. - PubMed
- Kiemer L, Cesareni G. Comparative interactomics: comparing apples and pears? Trends Biotechnol. 2007;25:448–454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P41 GM103314/GM/NIGMS NIH HHS/United States
- P41 GM109824/GM/NIGMS NIH HHS/United States
- P50 GM076547/GM/NIGMS NIH HHS/United States
- U54 GM103511/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases