The functional impact of the intestinal microbiome on mucosal immunity and systemic autoimmunity - PubMed (original) (raw)
Review
The functional impact of the intestinal microbiome on mucosal immunity and systemic autoimmunity
Randy S Longman et al. Curr Opin Rheumatol. 2015 Jul.
Abstract
Purpose of review: This review will highlight recent advances functionally linking the gut microbiome with mucosal and systemic immune cell activation underlying autoimmunity.
Recent findings: Dynamic interactions between the gut microbiome and environmental cues (including diet and medicines) shape the effector potential of the microbial organ. Key bacteria and viruses have emerged that, in defined microenvironments, play a critical role in regulating effector lymphocyte functions. The coordinated interactions between these different microbial kingdoms - including bacteria, helminths, and viruses (termed transkingdom interactions) - play a key role in shaping immunity. Emerging strategies to identify immunologically relevant microbes with the potential to regulate immune cell functions both at mucosal sites and systemically will likely define diagnostic and therapeutic targets.
Summary: The microbiome constitutes a critical microbial organ with coordinated interactions that shape host immunity.
Conflict of interest statement
Conflicts of interest
None
Figures
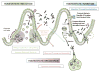
Figure 1. Microbial Regulation of Immunity and Autoimmunity
Homeostatic induction is maintained by sentinel microbes in which the barrier remains intact. Representative adherent microbes such as the mouse commensal segmented filamentous bacteria (SFB) tethers to the ileal mucosa and induces antigen-specific Th17 polarization. Similarly, key clostridial species promote the differentiation of induced Treg cells. Viruses, such as mouse norovirus (MNV), are sufficient to promote lymphocyte homeostasis in the lamina propria via an IFNα-dependent mechanism. Homeostatic inhibition is achieved by re-enforcing intestinal compartmentalization of microbes. Microbial activation of CX3CR1+ mononuclear phagocytes regulates group 3 innate lymphoid cells (ILC3) to produce the key cytokine IL-22 which promotes mucosal healing, anti-microbial peptide production, and modification of carbohydrates for bacteria. Homeostatic breakdown variably alters these mechanisms, resulting in trafficking of luminal microbes to mesenteric lymph nodes by CX3CR1+ MNPs, aberrant immunity to commensal microbiota, and subsequent systemic autoimmunity.
Similar articles
- How the Interplay Between the Commensal Microbiota, Gut Barrier Integrity, and Mucosal Immunity Regulates Brain Autoimmunity.
Antonini M, Lo Conte M, Sorini C, Falcone M. Antonini M, et al. Front Immunol. 2019 Aug 16;10:1937. doi: 10.3389/fimmu.2019.01937. eCollection 2019. Front Immunol. 2019. PMID: 31475000 Free PMC article. Review. - Host Factors of Favorable Intestinal Microbial Colonization.
Pirr S, Viemann D. Pirr S, et al. Front Immunol. 2020 Oct 7;11:584288. doi: 10.3389/fimmu.2020.584288. eCollection 2020. Front Immunol. 2020. PMID: 33117398 Free PMC article. Review. - The microbiome and autoimmune disease: Report from a Noel R. Rose Colloquium.
Barin JG, Tobias LD, Peterson DA. Barin JG, et al. Clin Immunol. 2015 Aug;159(2):183-8. doi: 10.1016/j.clim.2015.05.009. Epub 2015 May 20. Clin Immunol. 2015. PMID: 26003843 - Commensal microbiome effects on mucosal immune system development in the ruminant gastrointestinal tract.
Taschuk R, Griebel PJ. Taschuk R, et al. Anim Health Res Rev. 2012 Jun;13(1):129-41. doi: 10.1017/S1466252312000096. Anim Health Res Rev. 2012. PMID: 22853940 Review. - Enteric Immunity: Happy Gut, Healthy Animal.
Chase CCL. Chase CCL. Vet Clin North Am Food Anim Pract. 2018 Mar;34(1):1-18. doi: 10.1016/j.cvfa.2017.10.006. Vet Clin North Am Food Anim Pract. 2018. PMID: 29421027 Free PMC article. Review.
Cited by
- Compositional alterations of gut microbiota in children with primary nephrotic syndrome after initial therapy.
Kang Y, Feng D, Law HK, Qu W, Wu Y, Zhu GH, Huang WY. Kang Y, et al. BMC Nephrol. 2019 Nov 26;20(1):434. doi: 10.1186/s12882-019-1615-4. BMC Nephrol. 2019. PMID: 31771550 Free PMC article. - Efficacy of the Autoimmune Protocol Diet as Part of a Multi-disciplinary, Supported Lifestyle Intervention for Hashimoto's Thyroiditis.
Abbott RD, Sadowski A, Alt AG. Abbott RD, et al. Cureus. 2019 Apr 27;11(4):e4556. doi: 10.7759/cureus.4556. Cureus. 2019. PMID: 31275780 Free PMC article. - Mini-Review: Human Microbiome and Rheumatic Diseases.
Vural M, Gilbert B, Üstün I, Caglar S, Finckh A. Vural M, et al. Front Cell Infect Microbiol. 2020 Nov 10;10:491160. doi: 10.3389/fcimb.2020.491160. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33304855 Free PMC article. Review. - Reduced monocyte and macrophage TNFSF15/TL1A expression is associated with susceptibility to inflammatory bowel disease.
Richard AC, Peters JE, Savinykh N, Lee JC, Hawley ET, Meylan F, Siegel RM, Lyons PA, Smith KGC. Richard AC, et al. PLoS Genet. 2018 Sep 10;14(9):e1007458. doi: 10.1371/journal.pgen.1007458. eCollection 2018 Sep. PLoS Genet. 2018. PMID: 30199539 Free PMC article. - Proton pump Inhibitors and Risk of Enteric Infection in Inflammatory Bowel Disease: A Self-controlled Case Series.
Varma S, Trudeau SJ, Li J, Freedberg DE. Varma S, et al. Inflamm Bowel Dis. 2024 Jan 5;30(1):38-44. doi: 10.1093/ibd/izad035. Inflamm Bowel Dis. 2024. PMID: 36917215 Free PMC article.
References
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. - PubMed
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. - PubMed
Publication types
MeSH terms
Grants and funding
- T32 DK083256/DK/NIDDK NIH HHS/United States
- DK083256-02/DK/NIDDK NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- R01 DK103358/DK/NIDDK NIH HHS/United States
- K08 DK099381/DK/NIDDK NIH HHS/United States
- HHMI_/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials