Podocyte-Specific Deletion of Yes-Associated Protein Causes FSGS and Progressive Renal Failure - PubMed (original) (raw)
Podocyte-Specific Deletion of Yes-Associated Protein Causes FSGS and Progressive Renal Failure
Monica Schwartzman et al. J Am Soc Nephrol. 2016 Jan.
Abstract
FSGS is the most common primary glomerular disease underlying ESRD in the United States and is increasing in incidence globally. FSGS results from podocyte injury, yet the mechanistic details of disease pathogenesis remain unclear. This has resulted in an unmet clinical need for cell-specific therapy in the treatment of FSGS and other proteinuric kidney diseases. We previously identified Yes-associated protein (YAP) as a prosurvival signaling molecule, the in vitro silencing of which increases podocyte susceptibility to apoptotic stimulus. YAP is a potent oncogene that is a prominent target for chemotherapeutic drug development. In this study, we tested the hypothesis that podocyte-specific deletion of Yap leads to proteinuric kidney disease through increased podocyte apoptosis. Yap was selectively silenced in podocytes using Cre-mediated recombination controlled by the podocin promoter. Yap silencing in podocytes resulted in podocyte apoptosis, podocyte depletion, proteinuria, and an increase in serum creatinine. Histologically, features characteristic of FSGS, including mesangial sclerosis, podocyte foot process effacement, tubular atrophy, interstitial fibrosis, and casts, were observed. In human primary FSGS, we noted reduced glomerular expression of YAP. Taken together, these results suggest a role for YAP as a physiologic antagonist of podocyte apoptosis, the signaling of which is essential for maintaining the integrity of the glomerular filtration barrier. These data suggest potential nephrotoxicity with strategies directed toward inhibition of YAP function. Further studies should evaluate the role of YAP in proteinuric glomerular disease pathogenesis and its potential utility as a therapeutic target.
Keywords: focal segmental glomerulosclerosis; glomerular disease; podocyte.
Copyright © 2016 by the American Society of Nephrology.
Figures
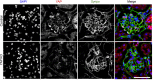
Figure 1.
Immunofluorescence labeling of YAP in glomeruli. Upper panels: YAP (red) colocalizes with the podocyte-specific marker synaptopodin (green) with additional nuclear expression colocalizing with DAPI (blue) detected. Lower panels: confirmation of reduction in YAP protein expression with podocyte-specific gene silencing. Scale bar, 50 _µ_m.
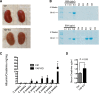
Figure 2.
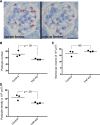
Kidney appearance and function with Yap gene silencing. (A) _Yap_-KO kidneys are of comparable size to controls (Ctrl), but exhibit a corrugated capsule at 12 weeks of age. (B) SDS-PAGE Coomassie-blue staining of standard BSA (numbers indicate _μ_g/_μ_l) and urine from _Yap_-KO and control mice. Proteinuria first appears between 5 and 6 weeks of age in _Yap_-KO mice. In each respective lane, 5 _μ_l of urine and 1 _μ_l of BSA standard were loaded. (C) Albumin-creatinine ratios demonstrating weekly progression of proteinuria in _Yap_-KO and control mice. At week 12, P<0.01. (D) Serum creatinine measurements showing a decrease in kidney function in _Yap_-KO mice. Ctrl, Pod-Cre-Yapflox/flox.
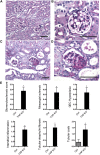
Figure 3.
PAS stainings of _Yap_-KO and control (Ctrl) mice at 12 weeks. (A,B) Ctrl kidney sections at low and high power. (C) _Yap_-KO mice with focal glomerulosclerosis and tubular dilatation and casts. (D) _Yap_-KO mouse with a lesion of focal segmental glomerulosclerosis. (E) Quantification of glomerular and tubular injury in 12-week-old mice. Scale bars, (A) and (C) 70 _µ_m; (B) and (D) 50 _µ_m.
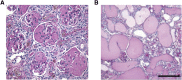
Figure 4.
Severe kidney disease in aged _Yap_-KO mice. (A) 32-week-old _Yap_-KO mouse PAS section with segmentally and globally sclerosed glomeruli. (B) 32-week-old _Yap_-KO mouse PAS section with diffuse tubular dilatation and proteinaceous casts. Scale bars, 50 _µ_m.
Figure 5.
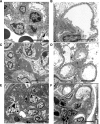
Electron microscopy images of _Yap_-KO mice. (A) Control with normal-appearing mesangial matrix. (B) Control with intact podocyte foot processes. (C) _Yap_-KO with moderate mesangial matrix accumulation (*). (D) _Yap_-KO with patchy foot process effacement. (E) _Yap_-KO with extensive FSGS with severe mesangial matrix expansion (**). (F) _Yap_-KO with diffuse podocyte foot process effacement. Scale bars, 3 _µ_m.
Figure 6.
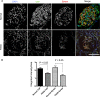
Podocyte apoptosis in _Yap_-KO mice. (A) PAS staining with apoptotic cell nucleus (arrow) in _Yap_-KO mouse at 12 weeks. (B) Terminal deoxynucleotidyl transferase–mediated 2′-deoxyuridine 5′-triphosphate nick-end labeling–positive nuclei in _Yap_-KO but not control glomeruli at 12 weeks. (C) Anti-cleaved poly adenosine diphosphate ribose polymerase (PARP) immunofluorescence staining in control and _Yap_-KO mice at 12 weeks. (D) Cleaved caspase 3 staining in control and _Yap_-KO mice at 7.5 weeks. Scale bars, (A) 20 _µ_m; (B-D) 50 _µ_m.
Figure 7.
Morphometric measurements. (A) Toluidine-blue staining showing quantification of podocyte number in adjacent epon sections 1-_µ_m thick. Only podocyte nuclei (asterisk) present in the sample section but not present in the look-up section (red arrows) were counted. Scale bar: 20 _µ_m. (B) Podocyte number was significantly reduced in _Yap_-KO mice at 12 weeks compared with the control. (C) Glomerular volumes were not significantly increased in _Yap_-KO compared with control (_P_=NS). (D) Podocyte density was reduced in _Yap_-KO mice compared with controls (_n_=3 mice, 30 total glomeruli per group).
Figure 8.
Expression of YAP in human glomeruli. (A) YAP expression in normal glomeruli compared with a patient with FSGS. Scale bar, 50 _µ_m. (B) Quantification of fluorescence intensity.
Similar articles
- The Hippo pathway regulator KIBRA promotes podocyte injury by inhibiting YAP signaling and disrupting actin cytoskeletal dynamics.
Meliambro K, Wong JS, Ray J, Calizo RC, Towne S, Cole B, El Salem F, Gordon RE, Kaufman L, He JC, Azeloglu EU, Campbell KN. Meliambro K, et al. J Biol Chem. 2017 Dec 22;292(51):21137-21148. doi: 10.1074/jbc.M117.819029. Epub 2017 Oct 5. J Biol Chem. 2017. PMID: 28982981 Free PMC article. - Nuclear exclusion of YAP exacerbates podocyte apoptosis and disease progression in Adriamycin-induced focal segmental glomerulosclerosis.
Zhuang Q, Li F, Liu J, Wang H, Tian Y, Zhang Z, Wang F, Zhao Z, Chen J, Wu H. Zhuang Q, et al. Lab Invest. 2021 Feb;101(2):258-270. doi: 10.1038/s41374-020-00503-3. Epub 2020 Nov 17. Lab Invest. 2021. PMID: 33203894 Free PMC article. - Podocyte YAP ablation decreases podocyte adhesion and exacerbates FSGS progression through α3β1 integrin.
Shao G, Xu J, Hu C, Jia W, Xu X, Gu Y, Zhang L, Zheng Z, Zhong J, Zhu S, Meng S, Zhao Z, Zhang Z, Liu J, Xu Y, Wu H. Shao G, et al. J Pathol. 2025 Jan;265(1):84-98. doi: 10.1002/path.6370. J Pathol. 2025. PMID: 39668547 - Protecting Podocytes: A Key Target for Therapy of Focal Segmental Glomerulosclerosis.
Campbell KN, Tumlin JA. Campbell KN, et al. Am J Nephrol. 2018;47 Suppl 1(Suppl 1):14-29. doi: 10.1159/000481634. Epub 2018 May 31. Am J Nephrol. 2018. PMID: 29852493 Free PMC article. Review. - Regulation of TRPC6 ion channels in podocytes - Implications for focal segmental glomerulosclerosis and acquired forms of proteinuric diseases.
Szabó T, Ambrus L, Zákány N, Balla G, Bíró T. Szabó T, et al. Acta Physiol Hung. 2015 Sep;102(3):241-51. doi: 10.1556/036.102.2015.3.2. Acta Physiol Hung. 2015. PMID: 26551740 Review.
Cited by
- Nephrin Suppresses Hippo Signaling through the Adaptor Proteins Nck and WTIP.
Keyvani Chahi A, Martin CE, Jones N. Keyvani Chahi A, et al. J Biol Chem. 2016 Jun 10;291(24):12799-12808. doi: 10.1074/jbc.M116.724245. Epub 2016 Mar 31. J Biol Chem. 2016. PMID: 27033705 Free PMC article. - Reciprocal regulation of YAP/TAZ by the Hippo pathway and the Small GTPase pathway.
Jang JW, Kim MK, Bae SC. Jang JW, et al. Small GTPases. 2020 Jul;11(4):280-288. doi: 10.1080/21541248.2018.1435986. Epub 2018 Apr 20. Small GTPases. 2020. PMID: 29457552 Free PMC article. Review. - Similar Biophysical Abnormalities in Glomeruli and Podocytes from Two Distinct Models.
Embry AE, Liu Z, Henderson JM, Byfield FJ, Liu L, Yoon J, Wu Z, Cruz K, Moradi S, Gillombardo CB, Hussain RZ, Doelger R, Stuve O, Chang AN, Janmey PA, Bruggeman LA, Miller RT. Embry AE, et al. J Am Soc Nephrol. 2018 May;29(5):1501-1512. doi: 10.1681/ASN.2017050475. Epub 2018 Mar 23. J Am Soc Nephrol. 2018. PMID: 29572404 Free PMC article. - Maintaining proteostasis under mechanical stress.
Höhfeld J, Benzing T, Bloch W, Fürst DO, Gehlert S, Hesse M, Hoffmann B, Hoppe T, Huesgen PF, Köhn M, Kolanus W, Merkel R, Niessen CM, Pokrzywa W, Rinschen MM, Wachten D, Warscheid B. Höhfeld J, et al. EMBO Rep. 2021 Aug 4;22(8):e52507. doi: 10.15252/embr.202152507. Epub 2021 Jul 26. EMBO Rep. 2021. PMID: 34309183 Free PMC article. Review. - Cross talk between the Crumbs complex and Hippo signaling in renal epithelial cells.
Michgehl U, Pavenstädt H, Vollenbröker B. Michgehl U, et al. Pflugers Arch. 2017 Aug;469(7-8):917-926. doi: 10.1007/s00424-017-2004-0. Epub 2017 Jun 13. Pflugers Arch. 2017. PMID: 28612137 Review.
References
- Alhassan A, Mohamed WZ, Alhaymed M: Patterns of childhood nephrotic syndrome in Aljouf region, Saudi Arabia. Saudi J Kidney Dis Transpl 24: 1050–1054, 2013 - PubMed
- Bonilla-Felix M, Parra C, Dajani T, Ferris M, Swinford RD, Portman RJ, Verani R: Changing patterns in the histopathology of idiopathic nephrotic syndrome in children. Kidney Int 55: 1885–1890, 1999 - PubMed
- Braden GL, Mulhern JG, O’Shea MH, Nash SV, Ucci AA, Jr, Germain MJ: Changing incidence of glomerular diseases in adults. Am J Kidney Dis 35: 878–883, 2000 - PubMed
- Gulati S, Sharma AP, Sharma RK, Gupta A: Changing trends of histopathology in childhood nephrotic syndrome. Am J Kidney Dis 34: 646–650, 1999 - PubMed
- Gulati S, Sural S, Sharma RK, Gupta A, Gupta RK: Spectrum of adolescent-onset nephrotic syndrome in Indian children. Pediatr Nephrol 16: 1045–1048, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK103022/DK/NIDDK NIH HHS/United States
- U54-MD007598/MD/NIMHD NIH HHS/United States
- T32 DK007757/DK/NIDDK NIH HHS/United States
- DK081617/DK/NIDDK NIH HHS/United States
- K08 DK081617/DK/NIDDK NIH HHS/United States
- U54 MD007598/MD/NIMHD NIH HHS/United States
LinkOut - more resources
Full Text Sources