Recessive osteogenesis imperfecta caused by missense mutations in SPARC - PubMed (original) (raw)
Case Reports
. 2015 Jun 4;96(6):979-85.
doi: 10.1016/j.ajhg.2015.04.021. Epub 2015 May 28.
Somayyeh Fahiminiya 2, Jacek Majewski 2; Care4Rare Canada Consortium; Martine Tétreault 2, Javad Nadaf 2, Peter Kannu 1, Etienne Sochett 3, Andrew Howard 4, Jennifer Stimec 5, Lucie Dupuis 1, Paul Roschger 6, Klaus Klaushofer 6, Telma Palomo 7, Jean Ouellet 7, Hadil Al-Jallad 7, John S Mort 7, Pierre Moffatt 7, Sergei Boudko 8, Hans-Peter Bächinger 8, Frank Rauch 9
Affiliations
- PMID: 26027498
- PMCID: PMC4457955
- DOI: 10.1016/j.ajhg.2015.04.021
Case Reports
Recessive osteogenesis imperfecta caused by missense mutations in SPARC
Roberto Mendoza-Londono et al. Am J Hum Genet. 2015.
Abstract
Secreted protein, acidic, cysteine-rich (SPARC) is a glycoprotein that binds to collagen type I and other proteins in the extracellular matrix. Using whole-exome sequencing to identify the molecular defect in two unrelated girls with severe bone fragility and a clinical diagnosis of osteogenesis imperfecta type IV, we identified two homozygous variants in SPARC (GenBank: NM_003118.3; c.497G>A [p.Arg166His] in individual 1; c.787G>A [p.Glu263Lys] in individual 2). Published modeling and site-directed mutagenesis studies had previously shown that the residues substituted by these mutations form an intramolecular salt bridge in SPARC and are essential for the binding of SPARC to collagen type I. The amount of SPARC secreted by skin fibroblasts was reduced in individual 1 but appeared normal in individual 2. The migration of collagen type I alpha chains produced by these fibroblasts was mildly delayed on SDS-PAGE gel, suggesting some overmodification of collagen during triple helical formation. Pulse-chase experiments showed that collagen type I secretion was mildly delayed in skin fibroblasts from both individuals. Analysis of an iliac bone sample from individual 2 showed that trabecular bone was hypermineralized on the material level. In conclusion, these observations show that homozygous mutations in SPARC can give rise to severe bone fragility in humans.
Copyright © 2015 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
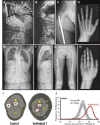
Phenotypic Findings in Individuals 1 and 2 (A and B) Anteroposterior and lateral views of the spine of individual 1 (age: 6 years), demonstrating severe scoliosis, kyphosis, and vertebral compression fractures. (C) Right hip and proximal femur of individual 1 (age: 14 years). The femoral neck has a very small diameter. An intramedullary rod has been inserted to treat repeated fractures and femoral bowing. (D) Hand and wrist radiograph of individual 1 (age: 14 years), showing metaphyseal lines from prior intravenous pamidronate infusions. The skeletal maturation (bone age) corresponds to chronological age. (E and F) Anteroposterior and lateral views of the spine of individual 2 (age: 6 years), showing mild scoliosis, kyphosis, and severe vertebral compression fractures. (G) The femurs of individual 2 (age: 6 years) have a small diameter and thin cortices, but are straight. A callus is visible on the right femur after a diaphyseal fracture. Metaphyseal lines resulting from pamidronate infusions are also visible. (H) Hand and wrist radiograph of individual 2 (age: 5 years), showing thin cortices of metacarpal bones but no dysplastic features. (I) Forearm peripheral quantitative computed tomography of individual 1 at the age of 14 years, compared to a healthy control of the same age and sex. The size of the two forearm cross-sections is similar, but the outer size of the radius (marked by arrows) is much lower in individual 1 (total cross-sectional area of the radius: 57 mm2; age- and sex-matched Z score: −3.9) than in the control individual (118 mm2, Z score +0.9). The percentage of fat (dark gray area, marked by an F) in the forearm cross-section is increased (48%), compared to the control (28%). The reverse is true for muscle (light gray area, marked by an M). (J) Bone mineralization density distribution in cancellous bone of individual 2. The curve labeled in gray represents pediatric reference data (mean and 95% confidence interval), as established previously in our laboratory.
Figure 2
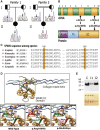
Confirmation of the Homozygous SPARC Mutations and Prediction of Their Impact on SPARC (A) Sanger sequencing results of individuals 1 and 2 and their parents. Individual 1 (II-1 in family 1) is homozygous for the c.497G>A variant, and both parents are heterozygous. Individual 2 (II-1 in family 2) is homozygous for the c.787G>A variant, and both parents are heterozygous. (B) Schematic representation of the coding exons of SPARC cDNA (GenBank:
NM_003118.3
) and of SPARC protein (GenBank:
NP_003109.1
). The locations of the exons are aligned relative to the regions of the SPARC protein that each exon encodes. The positions of the mutations reported in the present study are indicated. Abbreviations are as follows: S, signal peptide; Acidic, acidic domain; FS, follistatin-like domain; EC, extracellular collagen binding domain. (C) The mutated SPARC residues Arg166 and Glu263 and several adjoining residues at each site are perfectly conserved among species. (D) Modeling of the interaction between SPARC and collagen type I. Top: SPARC EC domain with the triple helical collagen type I alpha chains shown in light blue (alpha 1 chain) and dark blue (alpha 2 chain). Bottom: the critical regions of SPARC and collagen type I are magnified. The Phe residue of the alpha 1 chain is surrounded by the “Phe pocket” of SPARC. Arg166 and Glu263 form a salt bridge that is critical for maintaining the Phe pocket. When Arg166 or Glu263 are substituted by other residues, the loop conformation of the Phe pocket can not be maintained and collagen binding is impaired. (E) Immunoblot of SPARC protein in conditioned medium of skin fibroblasts from a control individual (C) and from individuals 1 (I1) and 2 (I2). The amount of SPARC in the medium is clearly lower in individual 1. Ponceau staining (bottom) shows equal loading in the control and individual 1, but somewhat lower loading for individual 2.
Figure 3
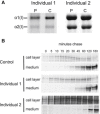
Collagen Type I Studies in Skin Fibroblasts (A) Biochemical analysis of collagen type I in the cell layer. Skin fibroblasts of individuals 1 and 2 (P) synthesize collagen type I alpha chains that have a very mild delay in migration, as compared to a control sample (C). (B) Pulse-chase analysis of collagen type I secretion. The bands in each set represent the collagen trimers that migrated into the gel under nonreducing conditions. In individuals 1 and 2, there is a delay in secretion of the type I collagen trimer.
Similar articles
- Biallelic variants in four genes underlying recessive osteogenesis imperfecta.
Hayat A, Hussain S, Bilal M, Kausar M, Almuzzaini B, Abbas S, Tanveer A, Khan A, Siddiqi S, Foo JN, Ahmad F, Khan F, Khan B, Anees M, Mäkitie O, Alfadhel M, Ahmad W, Umair M. Hayat A, et al. Eur J Med Genet. 2020 Aug;63(8):103954. doi: 10.1016/j.ejmg.2020.103954. Epub 2020 May 13. Eur J Med Genet. 2020. PMID: 32413570 - Mutations in WNT1 are a cause of osteogenesis imperfecta.
Fahiminiya S, Majewski J, Mort J, Moffatt P, Glorieux FH, Rauch F. Fahiminiya S, et al. J Med Genet. 2013 May;50(5):345-8. doi: 10.1136/jmedgenet-2013-101567. Epub 2013 Feb 23. J Med Genet. 2013. PMID: 23434763 - Expanding the phenotype of _SPARC_-related osteogenesis imperfecta: clinical findings in two patients with pathogenic variants in SPARC and literature review.
Durkin A, DeVile C, Arundel P, Bull M, Walsh J, Bishop NJ, Hupin E, Parekh S, Nadarajah R, Offiah AC, Calder A, Brock J, Baker D, Balasubramanian M. Durkin A, et al. J Med Genet. 2022 Aug;59(8):810-816. doi: 10.1136/jmedgenet-2021-107942. Epub 2021 Aug 30. J Med Genet. 2022. PMID: 34462290 Review. - SPARC/osteonectin in mineralized tissue.
Rosset EM, Bradshaw AD. Rosset EM, et al. Matrix Biol. 2016 May-Jul;52-54:78-87. doi: 10.1016/j.matbio.2016.02.001. Epub 2016 Feb 3. Matrix Biol. 2016. PMID: 26851678 Free PMC article. Review.
Cited by
- Update on the Genetics of Osteogenesis Imperfecta.
Jovanovic M, Marini JC. Jovanovic M, et al. Calcif Tissue Int. 2024 Dec;115(6):891-914. doi: 10.1007/s00223-024-01266-5. Epub 2024 Aug 11. Calcif Tissue Int. 2024. PMID: 39127989 Free PMC article. Review. - Cyclic bisphosphonate therapy reduces pain and improves physical functioning in children with osteogenesis imperfecta.
Garganta MD, Jaser SS, Lazow MA, Schoenecker JG, Cobry E, Hays SR, Simmons JH. Garganta MD, et al. BMC Musculoskelet Disord. 2018 Sep 24;19(1):344. doi: 10.1186/s12891-018-2252-y. BMC Musculoskelet Disord. 2018. PMID: 30249227 Free PMC article. - Two novel mutations in TMEM38B result in rare autosomal recessive osteogenesis imperfecta.
Lv F, Xu XJ, Wang JY, Liu Y, Asan, Wang JW, Song LJ, Song YW, Jiang Y, Wang O, Xia WB, Xing XP, Li M. Lv F, et al. J Hum Genet. 2016 Jun;61(6):539-45. doi: 10.1038/jhg.2016.11. Epub 2016 Feb 25. J Hum Genet. 2016. PMID: 26911354 - Creating an atlas of the bone microenvironment during oral inflammatory-related bone disease using single-cell profiling.
Fan Y, Lyu P, Bi R, Cui C, Xu R, Rosen CJ, Yuan Q, Zhou C. Fan Y, et al. Elife. 2023 Feb 1;12:e82537. doi: 10.7554/eLife.82537. Elife. 2023. PMID: 36722472 Free PMC article. - COL1A2 p.Gly1066Val variant identified in a Han Chinese family with osteogenesis imperfecta type I.
Wang M, Guo Y, Rong P, Xu H, Gong L, Deng H, Yuan L. Wang M, et al. Mol Genet Genomic Med. 2019 May;7(5):e619. doi: 10.1002/mgg3.619. Epub 2019 Mar 4. Mol Genet Genomic Med. 2019. PMID: 30829463 Free PMC article.
References
- Rauch F., Husseini A., Roughley P., Glorieux F.H., Moffatt P. Lack of circulating pigment epithelium-derived factor is a marker of osteogenesis imperfecta type VI. J. Clin. Endocrinol. Metab. 2012;97:E1550–E1556. - PubMed
- Roschger P., Paschalis E.P., Fratzl P., Klaushofer K. Bone mineralization density distribution in health and disease. Bone. 2008;42:456–466. - PubMed
- Fahiminiya S., Majewski J., Roughley P., Roschger P., Klaushofer K., Rauch F. Whole-exome sequencing reveals a heterozygous LRP5 mutation in a 6-year-old boy with vertebral compression fractures and low trabecular bone density. Bone. 2013;57:41–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous