Tau stabilizes microtubules by binding at the interface between tubulin heterodimers - PubMed (original) (raw)
Tau stabilizes microtubules by binding at the interface between tubulin heterodimers
Harindranath Kadavath et al. Proc Natl Acad Sci U S A. 2015.
Abstract
The structure, dynamic behavior, and spatial organization of microtubules are regulated by microtubule-associated proteins. An important microtubule-associated protein is the protein Tau, because its microtubule interaction is impaired in the course of Alzheimer's disease and several other neurodegenerative diseases. Here, we show that Tau binds to microtubules by using small groups of evolutionary conserved residues. The binding sites are formed by residues that are essential for the pathological aggregation of Tau, suggesting competition between physiological interaction and pathogenic misfolding. Tau residues in between the microtubule-binding sites remain flexible when Tau is bound to microtubules in agreement with a highly dynamic nature of the Tau-microtubule interaction. By binding at the interface between tubulin heterodimers, Tau uses a conserved mechanism of microtubule polymerization and, thus, regulation of axonal stability and cell morphology.
Keywords: Alzheimer's disease; NMR spectroscopy; Tau; chemical cross-linking; microtubule.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
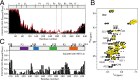
Tau binds microtubules through short evolutionary conserved sequence motifs. (A) 1H-15N NMR signal intensities of free Tau, _I_free, are compared with those of Tau bound to microtubules, I bound (Tau:tubulin heterodimer ratio of 1:3.6). The estimated error in _I_bound/I _f_ree according to the signal-to-noise ratio in the spectra was on average 0.08. Data for three-repeat Tau are shown as red line. The absence of repeat R2 in three-repeat Tau is indicated by vertical dashed lines. Tau’s domain organization is shown on top: I, insert; P, proline-rich region; R, repeat. (B) Superposition of 1H-15N CRINEPT-HMQC-TROSY spectra of Tau(208-324) in the free (black) and microtubule-bound (yellow) state acquired at 30 °C. The Tau(208-324):tubulin heterodimer ratio was 1:2. Selected resonances, which are strongly attenuated when Tau(208-324) is bound to microtubules, are labeled. (C) Signal intensity profile of Tau(208-324) bound to microtubules. NMR intensities were obtained from the spectra shown in B. At a Tau(208-324):tubulin heterodimer ratio of 1:2, Tau(208-324) is fully bound to microtubules (26). The detection of signals from residues in between the microtubule-binding motifs shows that these Tau residues remain flexible in the Tau–microtubule complex.
Fig. 2.
Tau competes with vinblastine for binding to tubulin. (A) Location of vinblastine (VB), colchicine (COL), and paclitaxel (PTX) on the 3D structure of the (Tc)2R complex (PDB ID code 1Z2B; ref. 31). Bound colchicine and vinblastine are shown by using the sphere model. Residues involved in paclitaxel binding (54) are highlighted in yellow. (B) Vinblastine competes with four-repeat Tau for binding to microtubules. Black bars represent the NMR line broadening observed in four-repeat Tau upon addition of microtubules (molar ratio of 2:1), whereas the gray line shows the corresponding values after addition of a 20-fold excess (with respect to four-repeat Tau) of vinblastine. (C) Electron micrograph of paclitaxel-stabilized microtubules after addition of vinblastine. The concentrations of Tau, microtubules and vinblastine were 10, 20, and 400 μM, respectively, in 50 mM sodium phosphate buffer, pH 6.8. (D) Competition between Tau fragments and vinblastine for binding to unpolymerized tubulin as probed by saturation-transfer difference NMR (SI Appendix, Figs. S4 and S5). Each of the six Tau fragments Tau(162-188) (blue), Tau(211-242) (purple), Tau(239-267) (light blue), Tau(265-290) (green), Tau(296-321) (orange), and Tau(368-402) (red) overlaps with one of the microtubule-binding motifs (Fig. 1 and SI Appendix, Fig. S1).
Fig. 3.
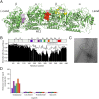
The repeat domain of Tau cross-links to α-tubulin. (A) Separation of cross-linked Tau-tubulin complexes by SDS/PAGE. (B) Map of cross-linked lysine res_i_dues. Cross-links are marked by dashed lines. Only cross-links from Tau to Lys336 and Lys338 in α-tubulin were observed. (C) Three-dimensional structure of the (Tc)2R complex (PDB ID code 1Z2B; ref. 31). Vinblastine is shown as red sphere model. Lys336 and Lys338 are highlighted in blue.
Fig. 4.
Tau binds at the interface between α-β-tubulin heterodimers. (A) Three-dimensional structure of the N terminus of a stathmin-like domain (green) bound to α-tubulin (55). Lys336 and Lys338 of α-tubulin, which were cross-linked to Tau (Fig. 3), are shown in red. Helix H10 and strand S9 that form the vinblastine binding pocket of α-tubulin are highlighted. (B) Average normalized signal intensity of residues 245–337 (black) in 1H-15N HSQC spectra of four-repeat Tau in the presence of unpolymerized tubulin and after addition of an x-fold molar excess of I19L with respect to Tau. For comparison, averaged normalized intensity values of residues 3–70 are shown in gray. Error bars show the SD over the selected residues.
Similar articles
- A functional role for intrinsic disorder in the tau-tubulin complex.
Melo AM, Coraor J, Alpha-Cobb G, Elbaum-Garfinkle S, Nath A, Rhoades E. Melo AM, et al. Proc Natl Acad Sci U S A. 2016 Dec 13;113(50):14336-14341. doi: 10.1073/pnas.1610137113. Epub 2016 Nov 23. Proc Natl Acad Sci U S A. 2016. PMID: 27911791 Free PMC article. - Mechanism of Tau-promoted microtubule assembly as probed by NMR spectroscopy.
Gigant B, Landrieu I, Fauquant C, Barbier P, Huvent I, Wieruszeski JM, Knossow M, Lippens G. Gigant B, et al. J Am Chem Soc. 2014 Sep 10;136(36):12615-23. doi: 10.1021/ja504864m. Epub 2014 Aug 27. J Am Chem Soc. 2014. PMID: 25162583 - Heterogeneous Tau-Tubulin Complexes Accelerate Microtubule Polymerization.
Li XH, Rhoades E. Li XH, et al. Biophys J. 2017 Jun 20;112(12):2567-2574. doi: 10.1016/j.bpj.2017.05.006. Biophys J. 2017. PMID: 28636913 Free PMC article. - Modulation of microtubule dynamics by drugs: a paradigm for the actions of cellular regulators.
Wilson L, Panda D, Jordan MA. Wilson L, et al. Cell Struct Funct. 1999 Oct;24(5):329-35. doi: 10.1247/csf.24.329. Cell Struct Funct. 1999. PMID: 15216890 Review. - Going new places using an old MAP: tau, microtubules and human neurodegenerative disease.
Garcia ML, Cleveland DW. Garcia ML, et al. Curr Opin Cell Biol. 2001 Feb;13(1):41-8. doi: 10.1016/s0955-0674(00)00172-1. Curr Opin Cell Biol. 2001. PMID: 11163132 Review.
Cited by
- A Novel Cre Recombinase Mouse Strain for Cell-Specific Deletion of Floxed Genes in Ribbon Synapse-Forming Retinal Neurons.
Suiwal S, Wartenberg P, Boehm U, Schmitz F, Schwarz K. Suiwal S, et al. Int J Mol Sci. 2024 Feb 5;25(3):1916. doi: 10.3390/ijms25031916. Int J Mol Sci. 2024. PMID: 38339191 Free PMC article. - Role of tau in the spatial organization of axonal microtubules: keeping parallel microtubules evenly distributed despite macromolecular crowding.
Méphon-Gaspard A, Boca M, Pioche-Durieu C, Desforges B, Burgo A, Hamon L, Piétrement O, Pastré D. Méphon-Gaspard A, et al. Cell Mol Life Sci. 2016 Oct;73(19):3745-60. doi: 10.1007/s00018-016-2216-z. Epub 2016 Apr 13. Cell Mol Life Sci. 2016. PMID: 27076215 Free PMC article. - Tau protein profiling in tauopathies: a human brain study.
Lantero-Rodriguez J, Camporesi E, Montoliu-Gaya L, Gobom J, Piotrowska D, Olsson M, Burmann IM, Becker B, Brinkmalm A, Burmann BM, Perkinton M, Ashton NJ, Fox NC, Lashley T, Zetterberg H, Blennow K, Brinkmalm G. Lantero-Rodriguez J, et al. Mol Neurodegener. 2024 Jul 19;19(1):54. doi: 10.1186/s13024-024-00741-9. Mol Neurodegener. 2024. PMID: 39026372 Free PMC article. - Accumulation of pTau231 at the Postsynaptic Density in Early Alzheimer's Disease.
Lilek J, Ajroud K, Feldman AZ, Krishnamachari S, Ghourchian S, Gefen T, Spencer CL, Kawles A, Mao Q, Tranovich JF, Jack CR, Mesulam MM, Reichard RR, Zhang H, Murray ME, Knopman D, Dickson DW, Petersen RC, Smith B, Ashe KH, Mielke MM, Nelson KM, Flanagan ME. Lilek J, et al. J Alzheimers Dis. 2023;92(1):241-260. doi: 10.3233/JAD-220848. J Alzheimers Dis. 2023. PMID: 36744338 Free PMC article. - It's all about tau.
Tapia-Rojas C, Cabezas-Opazo F, Deaton CA, Vergara EH, Johnson GVW, Quintanilla RA. Tapia-Rojas C, et al. Prog Neurobiol. 2019 Apr;175:54-76. doi: 10.1016/j.pneurobio.2018.12.005. Epub 2018 Dec 31. Prog Neurobiol. 2019. PMID: 30605723 Free PMC article. Review.
References
- Hirokawa N, Takemura R. Molecular motors and mechanisms of directional transport in neurons. Nat Rev Neurosci. 2005;6(3):201–214. - PubMed
- Kirschner M, Mitchison T. Beyond self-assembly: From microtubules to morphogenesis. Cell. 1986;45(3):329–342. - PubMed
- Mandelkow E, Mandelkow E-M. Microtubules and microtubule-associated proteins. Curr Opin Cell Biol. 1995;7(1):72–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources