Gene therapy for malignant glioma - PubMed (original) (raw)
Review
Gene therapy for malignant glioma
Hidehiro Okura et al. Mol Cell Ther. 2014.
Abstract
Glioblastoma multiforme (GBM) is the most frequent and devastating primary brain tumor in adults. Despite current treatment modalities, such as surgical resection followed by chemotherapy and radiotherapy, only modest improvements in median survival have been achieved. Frequent recurrence and invasiveness of GBM are likely due to the resistance of glioma stem cells to conventional treatments; therefore, novel alternative treatment strategies are desperately needed. Recent advancements in molecular biology and gene technology have provided attractive novel treatment possibilities for patients with GBM. Gene therapy is defined as a technology that aims to modify the genetic complement of cells to obtain therapeutic benefit. To date, gene therapy for the treatment of GBM has demonstrated anti-tumor efficacy in pre-clinical studies and promising safety profiles in clinical studies. However, while this approach is obviously promising, concerns still exist regarding issues associated with transduction efficiency, viral delivery, the pathologic response of the brain, and treatment efficacy. Tumor development and progression involve alterations in a wide spectrum of genes, therefore a variety of gene therapy approaches for GBM have been proposed. Improved viral vectors are being evaluated, and the potential use of gene therapy alone or in synergy with other treatments against GBM are being studied. In this review, we will discuss the most commonly studied gene therapy approaches for the treatment of GBM in preclinical and clinical studies including: prodrug/suicide gene therapy; oncolytic gene therapy; cytokine mediated gene therapy; and tumor suppressor gene therapy. In addition, we review the principles and mechanisms of current gene therapy strategies as well as advantages and disadvantages of each.
Keywords: Cytokine mediated; Gene therapy; Glioblastoma; Oncolytic; Prodrug suicide; Tumor suppressor gene.
Figures
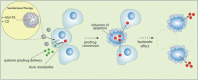
Figure 1
Strategy for suicide gene therapy. The aim of suicide gene therapy strategy is to increase the delivery of toxic metabolites to tumor cells and result in efficient cell death. Initially, a gene encoding a prodrug-activating enzyme is delivered by a tumor-targetting viral vector. Subsequent systemic administration of an inactive prodrug results in generation of a toxic metabolite and cell death of the transduced cells and non-trasduced bystander tumor cells (bystander effect) only at the tumor site.
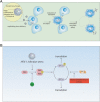
Figure 2
Strategy and mechanism for oncolytic gene therapy. (A); Oncolytic gene therapy employs replication-competent virus vectors capable of selective replication in target tumor cells. Spreading to new adjacent progeny cells occurs as the host cell is lysed and progeny virus is released. (B); Most viruses can replicate poorly in normal cells by a defense mechanism as follows. In response to viral infection, Protein Kinase R (PKR) in the host cells shut off protein synthesis by which PKR dimerizes and is inactivated by autophosphorylation resulting in the conversion of eukaryotic initiation factor-2 alpha (EIF-2α) into its inactive state following phosphorylation, which is required for translation initiation. Consequently, translation is arrested in the infected host cells as an anti-viral protective mechanism. However, the ICP34.5 in HSV-1 can overcome this defense by recruiting protein phosphatase-1α to dephosphorylate EIF-2α allowing protein synthesis to proceed. Therefore, when a deletion of γ34.5 gene is engineered, the HSV-1 mutant can no longer successfully proliferate in non-replicating cells. HSV-1 lacking ICP34.5 activity can only infect cells with defective PKR pathway. In tumor cells, PKR autophosphorylation is blocked due to Ras activation, permitting replication of viruses lacking the γ34.5 gene in tumor cells with hyper-activated Ras.
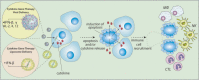
Figure 3
Strategy of cytokine mediated gene therapy. Cytokine mediated gene therapy involves tumor-selective gene transfer and in situ expression of various cytokine genes such as interleukin (IL) and interferon (IFN) capable of attracting immunocompetent cells such as macrophages (MΦ), natural killer cells (NK), and cytotoxic T lymphocytes (CTL) inducing immune response.
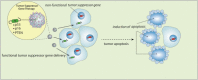
Figure 4
Strategy of tumor suppressor gene therapy. Tumor suppressor gene therapy aims to reprogram tumor cells by restoring the function of a tumor suppressor gene lost or functionally inactivated in cancer cells, subsequently inducing cell cycle arrest or apoptosis.
Similar articles
- Current Approaches for Glioma Gene Therapy and Virotherapy.
Banerjee K, Núñez FJ, Haase S, McClellan BL, Faisal SM, Carney SV, Yu J, Alghamri MS, Asad AS, Candia AJN, Varela ML, Candolfi M, Lowenstein PR, Castro MG. Banerjee K, et al. Front Mol Neurosci. 2021 Mar 11;14:621831. doi: 10.3389/fnmol.2021.621831. eCollection 2021. Front Mol Neurosci. 2021. PMID: 33790740 Free PMC article. Review. - Combinatorial approaches to effective therapy in glioblastoma (GBM): Current status and what the future holds.
Asija S, Chatterjee A, Yadav S, Chekuri G, Karulkar A, Jaiswal AK, Goda JS, Purwar R. Asija S, et al. Int Rev Immunol. 2022;41(6):582-605. doi: 10.1080/08830185.2022.2101647. Epub 2022 Aug 8. Int Rev Immunol. 2022. PMID: 35938932 Review. - The art of gene therapy for glioma: a review of the challenging road to the bedside.
Tobias A, Ahmed A, Moon KS, Lesniak MS. Tobias A, et al. J Neurol Neurosurg Psychiatry. 2013 Feb;84(2):213-22. doi: 10.1136/jnnp-2012-302946. Epub 2012 Sep 19. J Neurol Neurosurg Psychiatry. 2013. PMID: 22993449 Free PMC article. Review. - Gene therapy and virotherapy: novel therapeutic approaches for brain tumors.
Kroeger KM, Muhammad AK, Baker GJ, Assi H, Wibowo MK, Xiong W, Yagiz K, Candolfi M, Lowenstein PR, Castro MG. Kroeger KM, et al. Discov Med. 2010 Oct;10(53):293-304. Discov Med. 2010. PMID: 21034670 Free PMC article. Review. - Clinical trials using oncolytic viral therapy to treat adult glioblastoma: a progress report.
Lu VM, Shah AH, Vallejo FA, Eichberg DG, Luther EM, Shah SS, Komotar RJ, Ivan ME. Lu VM, et al. Neurosurg Focus. 2021 Feb;50(2):E3. doi: 10.3171/2020.11.FOCUS20860. Neurosurg Focus. 2021. PMID: 33524946
Cited by
- Identification of a novel disulfideptosis-related gene signature for prognostic implication in lower-grade gliomas.
Zhang F, Lv M, He Y. Zhang F, et al. Aging (Albany NY). 2024 Mar 27;16(7):6054-6067. doi: 10.18632/aging.205688. Epub 2024 Mar 27. Aging (Albany NY). 2024. PMID: 38546389 Free PMC article. - Gene therapy in glioblastoma multiforme: Can it be a role changer?
Rayati M, Mansouri V, Ahmadbeigi N. Rayati M, et al. Heliyon. 2024 Feb 24;10(5):e27087. doi: 10.1016/j.heliyon.2024.e27087. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38439834 Free PMC article. Review. - Electrospun Drug-Loaded and Gene-Loaded Nanofibres: The Holy Grail of Glioblastoma Therapy?
Louis L, Chee BS, McAfee M, Nugent M. Louis L, et al. Pharmaceutics. 2023 Jun 3;15(6):1649. doi: 10.3390/pharmaceutics15061649. Pharmaceutics. 2023. PMID: 37376095 Free PMC article. Review. - Systematic Review of Pediatric Brain Tumors in Neurofibromatosis Type 1: Status of Gene Therapy.
Thomas S, Bikeyeva V, Abdullah A, Radivojevic A, Abu Jad AA, Ravanavena A, Ravindra C, Igweonu-Nwakile EO, Ali S, Paul S, Yakkali S, Teresa Selvin S, Hamid P. Thomas S, et al. Cureus. 2022 Aug 13;14(8):e27963. doi: 10.7759/cureus.27963. eCollection 2022 Aug. Cureus. 2022. PMID: 36120213 Free PMC article. Review. - Against the Resilience of High-Grade Gliomas: Gene Therapies (Part II).
Giotta Lucifero A, Luzzi S. Giotta Lucifero A, et al. Brain Sci. 2021 Jul 23;11(8):976. doi: 10.3390/brainsci11080976. Brain Sci. 2021. PMID: 34439595 Free PMC article. Review.
References
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. - PubMed
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. - PubMed
- van den Bent MJ, Hegi ME, Stupp R. Recent developments in the use of chemotherapy in brain tumours. Eur J Cancer. 2006;42:582–588. - PubMed
- Kanu OO, Mehta A, Di C, Lin N, Bortoff K, Bigner DD, Yan H, Adamson DC. Glioblastoma multiforme: a review of therapeutic targets. Expert Opin Ther Targets. 2009;13:701–718. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources