Stromal miR-200s contribute to breast cancer cell invasion through CAF activation and ECM remodeling - PubMed (original) (raw)
doi: 10.1038/cdd.2015.78. Epub 2015 Jun 12.
Y Hou 2, G Yang 3, X Wang 4, S Tang 1, Y-E Du 1, L Yang 1, T Yu 3, H Zhang 1, M Zhou 1, S Wen 1, L Xu 1, M Liu 1
Affiliations
- PMID: 26068592
- PMCID: PMC4815985
- DOI: 10.1038/cdd.2015.78
Stromal miR-200s contribute to breast cancer cell invasion through CAF activation and ECM remodeling
X Tang et al. Cell Death Differ. 2016 Jan.
Abstract
The activation of cancer-associated fibroblasts (CAFs) is a key event in tumor progression, and alternative extracellular matrix (ECM) proteins derived from CAFs induce ECM remodeling and cancer cell invasion. Here we found that miR-200 s, which are generally downregulated in activated CAFs in breast cancer tissues and in normal fibroblasts (NFs) activated by breast cancer cells, are direct mediators of NF reprogramming into CAFs and of ECM remodeling. NFs with downregulated miR-200 s displayed the traits of activated CAFs, including accelerated migration and invasion. Ectopic expression of miR-200 s in CAFs at least partially restored the phenotypes of NFs. CAF activation may be governed by the targets of miR-200 s, Fli-1 and TCF12, which are responsible for cell development and differentiation; Fli-1 and TCF12 were obviously elevated in CAFs. Furthermore, miR-200 s and their targets influenced collagen contraction by CAFs. The upregulation of fibronectin and lysyl oxidase directly by miR-200 or indirectly through Fli-1 or TCF12 contributed to ECM remodeling, triggering the invasion and metastasis of breast cancer cells both in vitro and vivo. Thus, these data provide important and novel insights into breast CAF activation and ECM remodeling, which trigger tumor cell invasion.
Figures
Figure 1
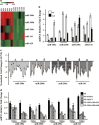
Expression of the miR-200 s is downregulated in CAFs. (a) Heat map illustrating hierarchical clustering of altered miR-200 family members in CAFs and paired NFs. (b) The expression of miR-200 s identified by miRNA array was confirmed using qRT-PCR in paired NFs and CAFs from three breast cancer patients. U6 was used as an internal control. The relative expression levels are shown as the fold change of CAFs compared with NFs (_n_=3). (c) The expression of the miR-200 family was evaluated by qRT-PCR in 20 paired NF and CAF samples that were freshly isolated from breast carcinoma tissues. U6 was used as an internal control. The data are shown as the normalized fold change of CAFs compared with NFs (_n_=3). (d) The expression of the miR-200 family was examined by qRT-PCR in NFs co-cultured with four different breast cancer cell lines (BT474, MCF-7, MDA-MB-453 and MDA-MB-468) for 15 or 30 days. U6 was used as an internal control. The data are shown as the normalized fold change of co-cultured NFs compared to NFs alone (_n_=3)
Figure 2
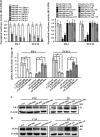
TCF12 and Fli-1 are directly regulated by miR-200 s. (a) Luciferase activity of CAFs co-transfected with different ratios of the indicated miR-200 s or control vector. TCF12 and Fli-1 reporters driven by wild-type 3'-UTRs (left panel) and TCF12 and Fli-1 reporters driven by 3'-UTRs with wild-type or mutated miR-200 s-binding sites (right panel) are shown. miR-Ctrl, non-miRNA-expressing control; wt UTR, wild-type 3′-UTR of TCF12 or Fli-1; mut UTR, mutant-binding sites for miR-200 s in the target mRNA. All the luciferase reporter assay results were normalized to Renilla luciferase, and the data are presented as the mean±S.D. (_n_=3; *P<0.05, ANOVA followed by the Student–Newman–Keuls test). (b) Lipofectamine 2000 was used to transiently transfect the miR-200 s mimics into CAFs or siRNA into NFs. Fli-1 and TCF12 expression was examined by qRT-PCR. _β_-Actin was used as an internal control. The data are presented as the mean±S.D. (_n_=3; *P<0.05, Student's t-test). (c and d) Western blot analysis of endogenous Fli-1 (c) and TCF12 (d) expression in the indicated fibroblasts. _β_-Actin was used as a loading control
Figure 3
Fli-1 and TCF12 expression levels are upregulated in breast tumor CAFs. (a and b) Western blot analysis of (a) endogenous Fli-1 and TCF12 protein expression in paired NFs and CAFs from three random patients or (b) total, nuclear and cytoplasmic Fli-1 and TCF12 protein expression in NFs co-cultured with four different breast cancer cell lines (BT474, MCF-7, MDA-MB-453 and MDA-MB-468) for 15 or 30 days. _β_-Actin, histone or tubulin was used as a loading control. (c) IHC staining for Fli-1 and TCF12 expression in breast specimens. The arrows indicate distinctly stained fibroblasts in the representative tumor tissues. Scale bars, 200 _μ_m. (d) Summary of IHC staining for normal, ductal carcinoma in situ and invasive ductal breast carcinoma samples. The nuclear staining intensities were categorized as low (+), medium (++), or high (+++) based on observations of 80% of the cell population. NG, not graded. (e) The Kaplan–Meier survival analysis revealed an association between poor prognosis in ER- breast cancer patients and TCF12 expression
Figure 4
Decreased expression of miR-200 s and high target gene expression promote the activation of NFs to form CAFs. (a–d) Western blot analysis of _α_-SMA and FAP expression in the indicated engineered CAFs or NFs. _β_-Actin was used as a loading control. (e) Transwell chamber analysis of the invasion of CAFs transfected with miR-200 s vectors or of NFs transfected with shRNAs targeting the miR-200 s. The data are presented as the mean±S.D. (_n_=3; *P<0.05, ANOVA followed by the Student–Newman–Keuls test). (f) Transwell chamber analysis of the invasion of CAFs transfected with shRNAs targeting Fli-1 or TCF12 and of NFs transfected with Fli-1 or TCF12 vectors. The data are presented as the mean±S.D. (_n_=3; *P<0.05, Student's t-test)
Figure 5
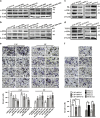
Activated CAFs generated in response to miR-200 s and their targets promote ECM remodeling. Fibroblasts were grown in a Col-I gel and cultured for 2 days. The ability of the indicated fibroblasts to elicit ECM remodeling was evaluated by contraction assays in Col-I gels. Representative images of Col-I gels are shown. The experiments were repeated at least three times, and the data are presented as the mean±S.D. (_n_>3; *P<0.05, Student's t-test). (a) Re-expression of the miR-200 s in CAFs reduced ECM remodeling. (b) Knockdown of miR-200 s in NFs enhanced the contractile activity of the indicated fibroblasts in a Col-I gel. (c) Decreasing Fli-1 or TCF12 expression using shRNA in CAFs reduced ECM remodeling. (d) Ectopic expression of Fli-1 or TCF12 in NFs increased ECM remodeling. (e) Restoring the expression of Fli-1 in CAF/miR-200c cells or TCF12 in CAF/miR-141 cells rescued the ECM remodeling of the indicated CAFs. (f) Silencing Fli-1 or TCF12 expression again in NF-sh/miR-200c or NF-sh/miR-141 cells attenuated their contractile activity in Col-I gels
Figure 6
miR-200 s and/or their targets Fli-1 and TCF12 are involved in the expression of ECM-associated genes in CAFs. (a) Heat map illustrating the hierarchical clustering of altered ECM-associated genes in CAFs and paired NFs. (b) qRT-PCR analysis of FN and LOX expression in 15 paired CAF and NF samples that were freshly isolated from breast tumor tissues. U6 was used as an internal control. The data are shown as the normalized fold change in CAFs compared with NFs. (c and d) Western blot analysis of FN and LOX expression in the indicated CAFs or NFs. (e) mRNA expression of FN and LOX as determined by qRT-PCR in the indicated CAFs or NFs. _β_-Actin was used as an internal control. The data are presented as the mean±S.D. (_n_=3; *P<0.05, Student's t-test). (f) Western blot analysis of FN and LOX expression in CAF-sh/TCF12 or NF/TCF12 cells. (g) LOX protein expression was analyzed by western blot after re-expressing Fli-1 in CAF/miR-200c cells or knocking down Fli-1 in NF-sh/miR-200c cells. (h) FN protein expression was evaluated by western blot after re-expressing TCF12 in CAF/miR-141 cells or knocking down TCF12 in NF-sh/miR-141 cells
Figure 7
miR-200 s and their targets Fli-1 and TCF12 promote breast cancer cell invasion in ECM. CAFs expressing vectors encoding miR-200 s were grown in a Col-I gel for 10 days and then treated with detergent extraction. MDA-MB-231 breast cancer cells were seeded onto the Col-I gel and cultured for 5 days. The architecture of the paraffin-embedded Col-I gel was visualized by PR staining, and the invasive cells in the ECM gel were visualized by H&E staining; representative images are shown (Scale bars, 100 _μ_m). (a–c) Matrices derived from the indicated fibroblasts were analyzed by PR staining for collagen deposition and orientation. (d–f) MDA-MB-231 cell invasion in the Col-I gel that was remodeled by the indicated fibroblasts. (g) MDA-MB-231 cell invasion in the Col-I gel that was remodeled by the indicated CAFs was determined after ectopically expressing Fli-1 in CAF/miR-200c cells or TCF12 in CAF/miR-141 cells. (h) MDA-MB-231 cell invasion in the Col-I gel that was remodeled by the indicated NFs was investigated after knocking down Fli-1 and miR-200c or TCF12 and miR-141 expression using specific shRNAs
Figure 8
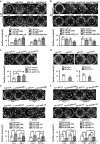
miR-200 s and their targets Fli-1 and TCF12 suppress the metastatic potential of malignant cells in vivo. MDA-MB-231 breast cancer cells were subcutaneously injected into nude mice in combination with CAF/miR-200 s, CAF/sh-Fli-1, CAF/sh-TCF12 or control cells; 45 days following injection, the mice were killed. Representative images (a) indicating tumor size in mice injected with MDA-MB-231 cells mixed with the indicated CAFs and (b) illustrating PR-stained tumor samples. Red, collagen fibers; Blue, nuclei. Scale bars, 100 _μ_m. Representative images of (c) a whole lung fixed in formalin for determining surface metastases and (d) an H&E-stained lung section confirming the presence of pulmonary metastases. The arrows indicate metastatic nodules. Graphs of the fold change in (e) the number of metastatic foci per lung, (f) the percentage of metastatic area to total lung area and (g) the area per lung section in five random sections per animal
Similar articles
- [Dectection and analysis of miRNA expression in breast cancer-associated fibroblasts].
Zeng Z, Hu P, Tang X, Zhang H, Du Y, Wen S, Liu M. Zeng Z, et al. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2014 Oct;30(10):1071-5. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2014. PMID: 25270211 Chinese. - Autocrine TGF-β1/miR-200s/miR-221/DNMT3B regulatory loop maintains CAF status to fuel breast cancer cell proliferation.
Tang X, Tu G, Yang G, Wang X, Kang L, Yang L, Zeng H, Wan X, Qiao Y, Cui X, Liu M, Hou Y. Tang X, et al. Cancer Lett. 2019 Jun 28;452:79-89. doi: 10.1016/j.canlet.2019.02.044. Epub 2019 Mar 6. Cancer Lett. 2019. PMID: 30851420 Free PMC article. - miR-101 represses lung cancer by inhibiting interaction of fibroblasts and cancer cells by down-regulating CXCL12.
Zhang J, Liu J, Liu Y, Wu W, Li X, Wu Y, Chen H, Zhang K, Gu L. Zhang J, et al. Biomed Pharmacother. 2015 Aug;74:215-21. doi: 10.1016/j.biopha.2015.08.013. Epub 2015 Aug 28. Biomed Pharmacother. 2015. PMID: 26349988 - Cancer-associated fibroblasts: a multifaceted driver of breast cancer progression.
Luo H, Tu G, Liu Z, Liu M. Luo H, et al. Cancer Lett. 2015 Jun 1;361(2):155-63. doi: 10.1016/j.canlet.2015.02.018. Epub 2015 Feb 17. Cancer Lett. 2015. PMID: 25700776 Review. - The role of cancer-associated fibroblasts in breast cancer pathobiology.
Jung YY, Kim HM, Koo JS. Jung YY, et al. Histol Histopathol. 2016 Apr;31(4):371-8. doi: 10.14670/HH-11-700. Epub 2015 Dec 2. Histol Histopathol. 2016. PMID: 26627101 Review.
Cited by
- Nanomedicine Strategies to Enhance Tumor Drug Penetration in Pancreatic Cancer.
Lu T, Prakash J. Lu T, et al. Int J Nanomedicine. 2021 Sep 15;16:6313-6328. doi: 10.2147/IJN.S279192. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34552327 Free PMC article. Review. - MicroRNAs in Metastasis and the Tumour Microenvironment.
Solé C, Lawrie CH. Solé C, et al. Int J Mol Sci. 2021 May 4;22(9):4859. doi: 10.3390/ijms22094859. Int J Mol Sci. 2021. PMID: 34064331 Free PMC article. Review. - Hsa-miR-134 suppresses non-small cell lung cancer (NSCLC) development through down-regulation of CCND1.
Sun CC, Li SJ, Li DJ. Sun CC, et al. Oncotarget. 2016 Jun 14;7(24):35960-35978. doi: 10.18632/oncotarget.8482. Oncotarget. 2016. PMID: 27166267 Free PMC article. - Crosstalk and plasticity driving between cancer-associated fibroblasts and tumor microenvironment: significance of breast cancer metastasis.
Zhang W, Wang J, Liu C, Li Y, Sun C, Wu J, Wu Q. Zhang W, et al. J Transl Med. 2023 Nov 17;21(1):827. doi: 10.1186/s12967-023-04714-2. J Transl Med. 2023. PMID: 37978384 Free PMC article. Review. - The Role of Cancer-Associated Fibroblasts and Extracellular Vesicles in Tumorigenesis.
Shoucair I, Weber Mello F, Jabalee J, Maleki S, Garnis C. Shoucair I, et al. Int J Mol Sci. 2020 Sep 17;21(18):6837. doi: 10.3390/ijms21186837. Int J Mol Sci. 2020. PMID: 32957712 Free PMC article. Review.
References
- Paszek MJ, Weaver VM. The tension mounts: mechanics meets morphogenesis and malignancy. J Mammary Gland Biol Neoplasia 2004; 9: 325–342. - PubMed
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005; 8: 241–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical