Cytoskeletal to Nuclear Strain Transfer Regulates YAP Signaling in Mesenchymal Stem Cells - PubMed (original) (raw)
Cytoskeletal to Nuclear Strain Transfer Regulates YAP Signaling in Mesenchymal Stem Cells
Tristan P Driscoll et al. Biophys J. 2015.
Abstract
Mechanical forces transduced to cells through the extracellular matrix are critical regulators of tissue development, growth, and homeostasis, and can play important roles in directing stem cell differentiation. In addition to force-sensing mechanisms that reside at the cell surface, there is growing evidence that forces transmitted through the cytoskeleton and to the nuclear envelope are important for mechanosensing, including activation of the Yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ) pathway. Moreover, nuclear shape, mechanics, and deformability change with differentiation state and have been likewise implicated in force sensing and differentiation. However, the significance of force transfer to the nucleus through the mechanosensing cytoskeletal machinery in the regulation of mesenchymal stem cell mechanobiologic response remains unclear. Here we report that actomyosin-generated cytoskeletal tension regulates nuclear shape and force transmission through the cytoskeleton and demonstrate the differential short- and long-term response of mesenchymal stem cells to dynamic tensile loading based on the contractility state, the patency of the actin cytoskeleton, and the connections it makes with the nucleus. Specifically, we show that while some mechanoactive signaling pathways (e.g., ERK signaling) can be activated in the absence of nuclear strain transfer, cytoskeletal strain transfer to the nucleus is essential for activation of the YAP/TAZ pathway with stretch.
Copyright © 2015 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
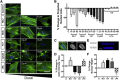
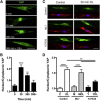
Cytoskeletal tension regulates nuclear and cytoskeletal morphology. (A) F-Actin (green) and DAPI (blue) images of cells seeded on glass and treated with different doses of ML7, Bleb, Y27632 (Y27), CytoD, or LPA (scale = 25 _μ_m). (B) Quantification of projected nuclear area on glass (mean ± SE, n > 85 cells/group). (C) Example of F-actin in the projected area of the nucleus (scale = 10 _μ_m), and (D) with quantification (mean ± SE, n > 85 cells/group; ML7 = 25 _μ_M, Y27632 = 10 _μ_M, CytoD = 10 _μ_M, LPA = 50 _μ_M). (E) Example X_-Z slices of nuclei (scale = 5 μ_m) used for quantification of nuclear height (F). (Mean ± SE, n > 13 cells/group; ∗_p < 0.05, ∗∗_p < 0.01, #p < 0.001 versus control; one-way ANOVA with Tukey’s post hoc testing.) To see this figure in color, go online.
Figure 2
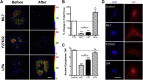
Traction force and YAP localization are regulated by ROCK and MLCK. (A) Traction stress maps for MSCs on 5-kPa polyacrylamide gels before and after addition of ML7, Y27632, or LPA (scale bar = 25 μ_m, units = Pa), (B) with quantification of % change in total force per cell (n = 14–20 cells per group, ∗_p < 0.05, ∗∗∗p < 0.001, one-way ANOVA with Tukey’s post hoc testing). Quantification of the nuclear/cytoplasmic YAP signal intensity for (C) MSCs seeded on glass and treated with ML7 (25 _μ_M), Y27632 (10 _μ_M), or LPA (50 _μ_M), and (D) with example epi-fluorescent images of YAP (red) and DAPI (blue) staining (scale bar = 25 _μ_m). To see this figure in color, go online.
Figure 3
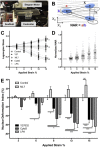
Contractility and nuclear prestrain regulate strain transmission to the nucleus. (A) Microtensile device used for stretch with simultaneous epi-fluorescent imaging of cell and nuclear deformation on scaffolds. (B) Schematic of deformation analysis indicating the nuclear aspect ratio (NAR) and the locations of nuclear triads in the deformed (x) and undeformed (X) states that were used for Lagrangian strain calculation. (C) Lagrangian strains including shear strain (_E_12), strain in the stretch direction (_E_11), and strain perpendicular to the stretch direction (_E_22). (D) Nuclear aspect ratio (NAR) for control cells normalized to the unstrained (0% strain) case. (E) Nuclear deformation index (NDI, which is NAR normalized to untreated control cells at the same strain, nNAR) for cells treated with ML7 (25 _μ_M), Y27632 (10 _μ_M), CytoD (2.5 _μ_M), or LPA (50 _μ_M). Nuclei that deform more than control have a positive NDI, and nuclei that deform less than control have a negative NDI. (Mean ± SE, n > 50 cells/group; two-way repeated measures ANOVA with Tukey’s post hoc testing.) To see this figure in color, go online.
Figure 4
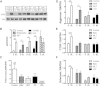
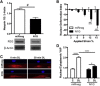
Contractility regulates response to dynamic stretch. (A) Western blots for phosphorylated ERK1/2 (pERK) and total ERK1/2 (ERK) for MSCs seeded on scaffolds and dynamically loaded (DL) to 3% strain at 1 Hz for 15 min with inhibition or activation of contractility (Bleb, 50 _μ_M, ML7: 25 _μ_M; Y27632, 10 _μ_M; CytoD, 2.5 _μ_M; LPA: 50 _μ_M). Densitometry for (B) Western blots (mean ± SE; n = 3/group) plotted as a ratio of pERK/ERK and (C) as the fold increase in pERK/ERK with loading. Gene expression measured by (D) RT-PCR for the cartilage marker aggrecan, (E) the growth factor CTGF, and (F) the tendon transcription factor scleraxis. Loading was administered for 6 h per day on two consecutive days; data represent the combined response of four independent experiments. (Mean ± SE, n = 9–12/group, two-way ANOVA with Tukey’s post hoc testing.)
Figure 5
YAP signaling is activated by dynamic stretch and requires strain transfer to the nucleus but not cytoskeletal tension. Representative _z_-projected images of (A) YAP staining and (B) quantification of the nuclear/cytoplasmic YAP for cells seeded on scaffold and dynamically stretched to 3% strain at 1 Hz for 0, 30, or 360 min or 360 min + 12 h of free-swelling culture (scale bar = 25 _μ_m; mean ± SE; n = 38–46 cells/group, two-way ANOVA with Tukey’s post hoc testing). Representative _z_-projected images of (C) YAP staining (green) with associated Actin (red) and DAPI (blue) with (D) quantification after 0 or 30 min of loading under control conditions or with the ROCK inhibitor Y27632 (10 μ_M) or the MLCK inhibitor ML7 (25 μ_M). (Mean ± SE, n = 26–34 cells/group, two-way ANOVA with Tukey’s post hoc testing; ∗∗_p < 0.01, ∗∗∗∗_p < 0.0001.) To see this figure in color, go online.
Figure 6
YAP signaling requires strain transfer to the nucleus through nesprin-1 giant. (A) Knockdown of nesprin-1 giant was performed using lentiviral delivery of miRNA, and knockdown was verified by dot blot for nesprin 1 after 1-MDa size filtration. (B) Quantification of the nuclear deformation index with knockdown of nesprin-1 giant compared to control cells (miRneg). Positive index indicates deformations larger than control, negative index indicates deformations less than control (mean ± SE, n = 146–149 cells/group). With less nesprin-1 giant to connect the nucleus to the cytoskeleton, smaller deformations occur. (C) Images of YAP (bottom) and DAPI (top) stained nuclei on aligned scaffolds with or without 30 min of dynamic tensile loading (DL, 3% strain; 1 Hz, scale = 25 μ_m) and with or without nestrpin-1 giant knockdown. (D) Quantification of the nuclear/cytoplasmic YAP for control (noted as “_C_”) or dynamically loaded (DL) cells. (Mean ± SE, n = 33–41 cells/group; # p < 0.05, ∗_p < 0.05, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.) To see this figure in color, go online.
Figure 7
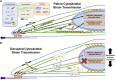
Schematic illustration of the differing routes of mechanotransduction. Mechanoactivation may occur via cell membrane-mediated mechanotransduction modules or through cytoskeletal/nuclear strain transfer-mediated mechanotransduction modules. (A) In baseline conditions, both pathways are likely operative. (B) When ROCK is inhibited with Y27632, a decrease in nuclear prestrain and depolymerization of actin is observed; when nesprin-1 giant levels are reduced, cytoskeletal strain transfer to the nucleus is likewise compromised. This leads to a loss in the cytoskeletal-to-nuclear strain transfer necessary for YAP activation, but does not completely abrogate signals originating as a result of tension at the cell membrane. To see this figure in color, go online.
Similar articles
- Role of YAP as a Mechanosensing Molecule in Stem Cells and Stem Cell-Derived Hematopoietic Cells.
Damkham N, Issaragrisil S, Lorthongpanich C. Damkham N, et al. Int J Mol Sci. 2022 Nov 23;23(23):14634. doi: 10.3390/ijms232314634. Int J Mol Sci. 2022. PMID: 36498961 Free PMC article. Review. - Vinculin promotes nuclear localization of TAZ to inhibit ECM stiffness-dependent differentiation into adipocytes.
Kuroda M, Wada H, Kimura Y, Ueda K, Kioka N. Kuroda M, et al. J Cell Sci. 2017 Mar 1;130(5):989-1002. doi: 10.1242/jcs.194779. Epub 2017 Jan 23. J Cell Sci. 2017. PMID: 28115535 - A Computational Model of YAP/TAZ Mechanosensing.
Sun M, Spill F, Zaman MH. Sun M, et al. Biophys J. 2016 Jun 7;110(11):2540-2550. doi: 10.1016/j.bpj.2016.04.040. Biophys J. 2016. PMID: 27276271 Free PMC article. - Control of cellular responses to mechanical cues through YAP/TAZ regulation.
Dasgupta I, McCollum D. Dasgupta I, et al. J Biol Chem. 2019 Nov 15;294(46):17693-17706. doi: 10.1074/jbc.REV119.007963. Epub 2019 Oct 8. J Biol Chem. 2019. PMID: 31594864 Free PMC article. Review.
Cited by
- Mechanoimmunology: Are inflammatory epigenetic states of macrophages tuned by biophysical factors?
Jain N, Lord JM, Vogel V. Jain N, et al. APL Bioeng. 2022 Aug 29;6(3):031502. doi: 10.1063/5.0087699. eCollection 2022 Sep. APL Bioeng. 2022. PMID: 36051106 Free PMC article. Review. - N-cadherin adhesive interactions modulate matrix mechanosensing and fate commitment of mesenchymal stem cells.
Cosgrove BD, Mui KL, Driscoll TP, Caliari SR, Mehta KD, Assoian RK, Burdick JA, Mauck RL. Cosgrove BD, et al. Nat Mater. 2016 Dec;15(12):1297-1306. doi: 10.1038/nmat4725. Epub 2016 Aug 15. Nat Mater. 2016. PMID: 27525568 Free PMC article. - Role of YAP as a Mechanosensing Molecule in Stem Cells and Stem Cell-Derived Hematopoietic Cells.
Damkham N, Issaragrisil S, Lorthongpanich C. Damkham N, et al. Int J Mol Sci. 2022 Nov 23;23(23):14634. doi: 10.3390/ijms232314634. Int J Mol Sci. 2022. PMID: 36498961 Free PMC article. Review. - Descemet's Membrane Biomimetic Microtopography Differentiates Human Mesenchymal Stem Cells Into Corneal Endothelial-Like Cells.
Gutermuth A, Maassen J, Harnisch E, Kuhlen D, Sauer-Budge A, Skazik-Voogt C, Engelmann K. Gutermuth A, et al. Cornea. 2019 Jan;38(1):110-119. doi: 10.1097/ICO.0000000000001765. Cornea. 2019. PMID: 30308581 Free PMC article. - Universally Conserved Relationships between Nuclear Shape and Cytoplasmic Mechanical Properties in Human Stem Cells.
Lozoya OA, Gilchrist CL, Guilak F. Lozoya OA, et al. Sci Rep. 2016 Mar 15;6:23047. doi: 10.1038/srep23047. Sci Rep. 2016. PMID: 26976044 Free PMC article.
References
- Engler A.J., Sen S., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. - PubMed
- McBeath R., Pirone D.M., Chen C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell. 2004;6:483–495. - PubMed
- Angele P., Schumann D., Kujat R. Cyclic, mechanical compression enhances chondrogenesis of mesenchymal progenitor cells in tissue engineering scaffolds. Biorheology. 2004;41:335–346. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AR050950/AR/NIAMS NIH HHS/United States
- R01 AR056624/AR/NIAMS NIH HHS/United States
- R01 EB002425/EB/NIBIB NIH HHS/United States
- R01 EB02425/EB/NIBIB NIH HHS/United States
- T32 AR007132/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous