Increased expression of NAPDH oxidase 4 in systemic sclerosis dermal fibroblasts: regulation by transforming growth factor β - PubMed (original) (raw)
Increased expression of NAPDH oxidase 4 in systemic sclerosis dermal fibroblasts: regulation by transforming growth factor β
Sonsoles Piera-Velazquez et al. Arthritis Rheumatol. 2015 Oct.
Abstract
Objective: Systemic sclerosis (SSc) is characterized by severe and often progressive fibrosis of the skin and multiple internal organs. The mechanisms responsible for these alterations remain obscure, although excessive reactive oxygen species (ROS)-mediated oxidative stress has been implicated. NOX-4 is 1 of 7 isoforms of NADPH oxidase responsible for the generation of ROS. The purpose of this study was to examine NOX-4 expression in skin and cultured dermal fibroblasts from SSc patients and to examine its regulation by transforming growth factor β1 (TGFβ1).
Methods: NOX-4 was assessed in normal and SSc skin by immunohistologic analysis and in normal and SSc cultured dermal fibroblasts by quantitative polymerase chain reaction analysis, fluorescence microscopy, and Western blotting. ROS levels were assessed by fluorescence measurement of H2 O2 production. Specific kinase inhibitors were used to study the TGFβ1 signaling involved in NOX-4 stimulation. NOX-4 inhibition/down-regulation was induced with a selective NOX-4 small-molecule inhibitor and NOX-4 small interfering RNA (siRNA).
Results: In contrast with normal skin fibroblasts, those from SSc skin showed intense NOX-4 staining. Cultured SSc fibroblasts displayed increased NOX-4 expression. TGFβ1 caused potent NOX-4 protein and messenger RNA stimulation in normal and SSc fibroblasts, which was mediated by the protein kinase Cδ (PKCδ) and Smad2/3 pathways. NOX-4 knockdown in SSc fibroblasts reduced the production of ROS and lowered the expression of type I collagen.
Conclusion: NOX-4 expression and production were found to be constitutively elevated in SSc skin and cultured SSc dermal fibroblasts. TGFβ1 stimulated NOX-4 expression in normal and SSc fibroblasts through PKCδ and Smad2/3 signaling pathways. A small-molecule NOX-4 inhibitor decreased collagen and fibronectin production by normal and SSc fibroblasts, and NOX-4 siRNA knockdown reduced ROS and collagen production by SSc fibroblasts. These results demonstrate the involvement of NOX-4 in SSc-associated fibrosis and indicate NOX-4 inhibitors as novel therapeutic approaches for SSc.
© 2015, American College of Rheumatology.
Figures
Figure 1
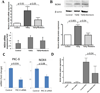
A. Immunofluorescence analysis of NOX4 protein levels in cultured normal and SSc dermal fibroblasts. Representative NOX4 immunofluorescence in confluent cultures of one normal and one SSc dermal fibroblast cell line. B. Western blot analysis of equal amounts of cell extracts from three normal and three SSc dermal fibroblasts probed with a specific NOX4 antibody (upper panel). The middle panel shows the same gel stained with comassie blue which was used as loading control for protein concentrations (middle panel). The bar graph (lower panel) represents the average NOX4 protein levels from 3 normal and 4 SSc cultured dermal fibroblast cell lines corrected for protein loading.* p value = 0.018. AU= arbitrary units of fluorescence. C. Immunohistological staining of normal and SSc skin tissues for NOX4 epitopes. The images shown are representative of images obtained from 3 samples of normal skin and 4 samples of affected SSc skin. Note intensely-stained NOX4 epitopes in numerous fibroblastic cells (black arrows) present only in SSc tissues (Magnification 10×). The insets show greater magnification images (Magnification 40×) of NOX4-positive cells in the SSc skin samples.
Figure 2
A. NOX4 mRNA expression levels assessed in triplicate by Real Time PCR analysis in four normal dermal fibroblast cell lines under treatment with TGF-β1 and rottlerin. GAPDH was used as endogenous control. Lower panel shows the effect of TGF-β1 and rottlerin on three SSc cell lines. B. Representative Western blot analysis of NOX4 and β-actin (as protein control) using cell extracts from four normal dermal fibroblast cell lines treated with TGF-β1 alone or TGF-β1 plus rottlerin. Bar graph represents NOX4 protein levels following correction for the intensity of the β actin band. C. Bar graphs of PKC-δ and NOX4 mRNA expression by normal dermal fibroblasts following treatment with specific siRNA against PKC-δ. The data shown are the average of the siRNA effect on three normal dermal fibroblast cell lines. D. Bar graph of PKC-δ expression by normal dermal fibroblasts following treatment with TGF-β1 and a specific siRNA against PKC-δ. The data shown are the average of duplicate experiments examining the PKC-δ siRNA effect on two normal dermal fibroblast cell lines.
Figure 3
Real Time PCR analysis of NOX4 expression (A) and protein levels of NOX4 (B) by normal dermal fibroblasts under treatment with either TGF-β1 alone or with TGF-β1 plus either imatinib mesylate (Imat; cAbl inhibitor) or SB4315421 (SB43; Smad2/3 specific inhibitor). The results are the average of three separate experiments with three different cell lines. * p value < 0.05. A representative Western blot is shown in the inset.
Figure 4
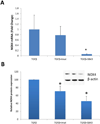
Fluorescence microscopy images of ROS levels in normal dermal fibroblasts treated with either TGF-β1 alone or TGF-β1 plus the NOX4/NOX1 small molecule inhibitor GKT137831 (GKT). The bar graph (lower panel) represents corrected total cell fluorescence (CTCF) of relative ROS production of TGF-β1 or TGF-β1 plus GKT inhibitor vs untreated cells in four separate experiments with two normal dermal fibroblast cell lines.* p value = 0.005, ** p value = 0.02.
Figure 5
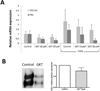
A. Real Time PCR analysis of COL1A1 and FN1 expression by normal dermal fibroblasts treated with increasing concentration of the NOX4/NOX1 specific inhibitor GKT137831 (GKT) and either culture media alone or culture media containing TGF-β1. The data shown are the average of the results obtained in three separate experiments performed with a normal cell line. B. Representative image of a Western blot analysis for secreted collagen Type I from normal dermal fibroblasts treated with 10 µM GKT. The bar graph shows the average results obtained with four different cell lines.
Figure 6
A. Bar graphs of NOX4 mRNA expression by SSc dermal fibroblasts following treatment with specific siRNA against NOX4. The data shown are the average of the siRNA effect on four SSc dermal fibroblast cell lines * p value < 0.05. B. Fluorescence microscopy images and quantification of ROS levels in SSc dermal fibroblasts following treatment with specific siRNA against NOX4. The graph represents the average intensity of fluorescence (CTCF) of two SSc dermal fibroblast cell lines examined in duplicate * p value < 0.05. C. Western blot analysis of secreted collagen Type I from the culture media of SSc dermal fibroblasts following NOX4 siRNA knockdown. Control cells were transfected with a scrambled inactive siRNA. The bar graph shows the average of results obtained with three separate SSc dermal fibroblast cell lines * p value < 0.01.
Similar articles
- A reactive oxygen species-mediated loop maintains increased expression of NADPH oxidases 2 and 4 in skin fibroblasts from patients with systemic sclerosis.
Spadoni T, Svegliati Baroni S, Amico D, Albani L, Moroncini G, Avvedimento EV, Gabrielli A. Spadoni T, et al. Arthritis Rheumatol. 2015 Jun;67(6):1611-22. doi: 10.1002/art.39084. Arthritis Rheumatol. 2015. PMID: 25707572 - Oxidative stress in scleroderma: maintenance of scleroderma fibroblast phenotype by the constitutive up-regulation of reactive oxygen species generation through the NADPH oxidase complex pathway.
Sambo P, Baroni SS, Luchetti M, Paroncini P, Dusi S, Orlandini G, Gabrielli A. Sambo P, et al. Arthritis Rheum. 2001 Nov;44(11):2653-64. doi: 10.1002/1529-0131(200111)44:11<2653::aid-art445>3.0.co;2-1. Arthritis Rheum. 2001. PMID: 11710721 - NADPH oxidase-2 is a key regulator of human dermal fibroblasts: a potential therapeutic strategy for the treatment of skin fibrosis.
Zhang GY, Wu LC, Dai T, Chen SY, Wang AY, Lin K, Lin DM, Yang JQ, Cheng B, Zhang L, Gao WY, Li ZJ. Zhang GY, et al. Exp Dermatol. 2014 Sep;23(9):639-44. doi: 10.1111/exd.12479. Exp Dermatol. 2014. PMID: 24981855 - Regulation of connective tissue synthesis in systemic sclerosis.
Varga J, Bashey RI. Varga J, et al. Int Rev Immunol. 1995;12(2-4):187-99. doi: 10.3109/08830189509056712. Int Rev Immunol. 1995. PMID: 7650421 Review.
Cited by
- Higher gamma-glutamyl transferase levels are associated with an increased risk of incident systemic sclerosis: a nationwide population-based study.
Kwon OC, Han K, Park MC. Kwon OC, et al. Sci Rep. 2023 Dec 11;13(1):21878. doi: 10.1038/s41598-023-49183-1. Sci Rep. 2023. PMID: 38072855 Free PMC article. - Abrogation of transforming growth factor-β-induced tissue fibrosis in mice with a global genetic deletion of Nox4.
Wermuth PJ, Mendoza FA, Jimenez SA. Wermuth PJ, et al. Lab Invest. 2019 Apr;99(4):470-482. doi: 10.1038/s41374-018-0161-1. Epub 2018 Nov 23. Lab Invest. 2019. PMID: 30470772 Free PMC article. - Phosphodiesterase 4 is overexpressed in keloid epidermal scars and its inhibition reduces keratinocyte fibrotic alterations.
Milara J, Ribera P, Marín S, Montero P, Roger I, Cortijo J. Milara J, et al. Mol Med. 2024 Sep 2;30(1):134. doi: 10.1186/s10020-024-00906-8. Mol Med. 2024. PMID: 39223490 Free PMC article. - Oxidative stress promotes fibrosis in systemic sclerosis through stabilization of a kinase-phosphatase complex.
Zhang R, Kumar GS, Hansen U, Zoccheddu M, Sacchetti C, Holmes ZJ, Lee MC, Beckmann D, Wen Y, Mikulski Z, Yang S, Santelli E, Page R, Boin F, Peti W, Bottini N. Zhang R, et al. JCI Insight. 2022 Apr 22;7(8):e155761. doi: 10.1172/jci.insight.155761. JCI Insight. 2022. PMID: 35451370 Free PMC article. - Endothelial to Mesenchymal Transition: Role in Physiology and in the Pathogenesis of Human Diseases.
Piera-Velazquez S, Jimenez SA. Piera-Velazquez S, et al. Physiol Rev. 2019 Apr 1;99(2):1281-1324. doi: 10.1152/physrev.00021.2018. Physiol Rev. 2019. PMID: 30864875 Free PMC article. Review.
References
- Jimenez SA, Derk CT. Following the molecular pathways toward an understanding of the pathogenesis of systemic sclerosis. Ann Intern Med. 2004;140:37–50. - PubMed
- Katsumoto TR, Whitfield ML, Connolly MK. The pathogenesis of systemic sclerosis. Annual Rev Pathol. 2011;6:509–537. - PubMed
- Balbir-Gurman A, Braun-Moscovici Y. Scleroderma-new aspects in pathogenesis and treatment. Best Pract Res Clin Rheumatol. 2012;26:13–24. - PubMed
- Denton CP, Black CM, Abraham DJ. Mechanisms and consequences of fibrosis in systemic sclerosis. Nat Clin Pract Rheumatol. 2006;2:134–144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources