The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna - PubMed (original) (raw)
The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna
Saad S Dahham et al. Molecules. 2015.
Abstract
The present study reports a bioassay-guided isolation of β-caryophyllene from the essential oil of Aquilaria crassna. The structure of β-caryophyllene was confirmed using FT-IR, NMR and MS. The antimicrobial effect of β-caryophyllene was examined using human pathogenic bacterial and fungal strains. Its anti-oxidant properties were evaluated by DPPH and FRAP scavenging assays. The cytotoxicity of β-caryophyllene was tested against seven human cancer cell lines. The corresponding selectivity index was determined by testing its cytotoxicity on normal cells. The effects of β-caryophyllene were studied on a series of in vitro antitumor-promoting assays using colon cancer cells. Results showed that β-caryophyllene demonstrated selective antibacterial activity against S. aureus (MIC 3 ± 1.0 µM) and more pronounced anti-fungal activity than kanamycin. β-Caryophyllene also displayed strong antioxidant effects. Additionally, β-caryophyllene exhibited selective anti-proliferative effects against colorectal cancer cells (IC50 19 µM). The results also showed that β-caryophyllene induces apoptosis via nuclear condensation and fragmentation pathways including disruption of mitochondrial membrane potential. Further, β-caryophyllene demonstrated potent inhibition against clonogenicity, migration, invasion and spheroid formation in colon cancer cells. These results prompt us to state that β-caryophyllene is the active principle responsible for the selective anticancer and antimicrobial activities of A. crassnia. β-Caryophyllene has great potential to be further developed as a promising chemotherapeutic agent against colorectal malignancies.
Keywords: anti-cancer; anti-clonogenic; apoptosis; colorectal cancer; nuclear fragmentation; β-caryophyllene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Figure 1
Schematic diagram showing the bioassay (anti-proliferative assay)-guided isolation of β-caryophyllene from the essential oils of Aquilaria crassna.
Figure 2
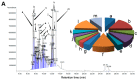
Gas chromatograpic analysis of Aquilaria crassna and β-caryophyllene. (A) Chemical characterization of the essential oil of Aquilaria crassna by GC-MS. The pie charts depict the relative chemical composition of the sub-fractions. Refer to Table S1 in the Supporting Information for the details of the peaks identified in the chromatogram; (B) The major peak corresponds to the the active principle, identified as β-caryophyllene, isolated from the essential oil of Aquilaria crassna.
Figure 2
Gas chromatograpic analysis of Aquilaria crassna and β-caryophyllene. (A) Chemical characterization of the essential oil of Aquilaria crassna by GC-MS. The pie charts depict the relative chemical composition of the sub-fractions. Refer to Table S1 in the Supporting Information for the details of the peaks identified in the chromatogram; (B) The major peak corresponds to the the active principle, identified as β-caryophyllene, isolated from the essential oil of Aquilaria crassna.
Figure 3
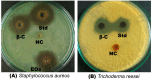
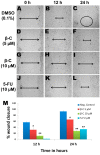
Antimicrobial effect of β-caryophyllene. (A) Antibacterial effect of β-caryophyllene (β-C) on Staphylococcus aureus (Std = Kanamycin; EOs = essential oil of Aquilaria crassna; NC = negative control); (B) Antifungal effect of β-caryophyllene (β-C) on Trichoderma reesei (Std = Kanamycin; NC = negative control).
Figure 4
Effect of β-caryophyllene on the cellular morphology of human cancer and normal cell lines. Photomicrographic images of cancer cell lines, taken under an inverted phase-contrast microscope at 200× magnification using a digital camera at 48 h after treatment with β-caryophyllene.
Figure 5
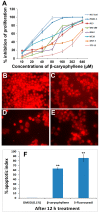
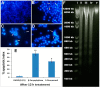
(A) Dose-dependent anti-proliferative effect of β-caryophyllene on, HCT 116, PANC-1, HT-29, MCF-7, PC3, K562, ME-180 and NIH/3T3-L1 cell lines was assessed by MTT-assay (values are represented as mean ± SD, n = 3); (B) Rhodamine 123 stained photomicrographic images of HCT 116 cells treated with vehicle (0.1% DMSO); (C) Rhodamine 123 stained photomicrographic images of HCT 116 cells treated with β-caryophyllene (10 µM) for 6 h; (D) Rhodamine 123 stained photomicrographic images of HCT 116 cells treated with β-caryophyllene (10 µM) for 12 h; (E) Rhodamine 123 stained photomicrographic images of HCT 116 cells treated with 5-flourouracil (10 µM) for 12 h; (F) Graphical representation of percentage of apoptotic indices. The apoptotic index for each test group was expressed as a percentage of the ratio of number of unstained cells to the total number of cell in 10 different microscopic fields. Values are presented as mean ± SD (n = 10), ** represents p < 0.01.
Figure 6
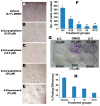
Photomicrographs depicting images of HCT 116 cells with Hoechst 33258 staining (A) Cells treated with vehicle (0.1% DMSO). The vehicle-treated cells revealed an intact cell membrane with an evenly distributed nucleus in cytosol; (B) Cells after 6 h of β-caryophyllene (10 µM) treatment. Cells treated with β-caryophyllene displayed early stage apoptotic symptoms such as membrane blebbing and chromatin condensation (arrows); (C) Cells after 12 h of β-caryophyllene (10 µM) treatment. The arrows indicate the advanced staged apoptotic signs such as of nuclear dissolution including the half-moon (crescent) shaped apoptotic nuclei. In addition, at several places, the arrows mark chromatin breakdown and fragmentation; (D) Cells treated with standard reference, 5-flourouracil (10 µM) also exhibited significant induction of apoptosis in the cells; (E) Graphical representation of percentage of apoptotic indices for HCT 116 and PANC-1 cells. The apoptotic index for each test group was expressed as a percentage of the ratio of number of apoptotic cells to the total number of cell in 10 different microscopic fields. Values are presented as mean ± SD (n = 10), * represents p < 0.05 and ** represents p < 0.01; (F) Effect of β-caryophyllene (10 µM) on DNA fragmentation in HCT 116 cells after 24 h treatment. (i). The standard DNA ladder; (ii). The DNA fragmentation pattern of HCT 116 cells treated with 5-flourouracil (10 µM); (iii). DNA fragmentation pattern of HCT 116 cells treated with β-caryophyllene (5 µM); (iv). DNA fragmentation pattern of HCT 116 cells treated with β-caryophyllene (10 µM); (v). DNA fragmentation pattern of HCT 116 cells treated with 0.1% DMSO (negative control).
Figure 7
Due to the successful migration of HCT 116 cells in the untreated group (negative control), the wound is almost closed after 24 h (A–C), whereas in the β-caryophyllene-treated monolayer, the wound remained open even after 24 h incubation. β-Caryophyllene (5 μM) caused a significant inhibition of HCT 116 cell migration (D–F). Interestingly, even at a sub-cytotoxic concentration (10 μM), the compound caused significant inhibition of migration (G–I). The results can be compared with those of the standard reference 5-FU (J–L). Graphical representation (M) of the time and dose and time-dependent inhibitory effect of β-caryophyllene on migration of HCT 116 (values are in mean ± SD, n = 6, * p < 0.1, ** p < 0.005).
Figure 8
(A) Photomicrographs of HCT 116 cells invading the matrigel barrier. The negative control group showed a large number of invaded cells; (B‒D) Photomicrographs of HCT 116 cells showing the anti-invasion effect of β-caryophyllene (6.25, 12.5 and 25 µM, respectively) on a matrigel matrix; (E) Photomicrographic images of HCT 116 cells showing the anti-invasion effect of 5-flourouracil (10 µM) on a matrigel matrix; (F) Graphical representation of mean number of cells invaded per field of view, after counting 10 microscopic fields of view for triplicate wells. In untreated wells, the population of the cells invaded through the matrigel was significantly more than that of the treated wells (*p < 0.05, ** p < 0.01); (G) Effect of β-caryophyllene on survival of HCT 116 colonies in a colony formation assay. The picture clearly depicts the strong anti-clonogenic effect of β-caryophyllene on colonies of the cancer cells; (H) The graphical representation illustrates the percentage of plating efficiencies after the treatment of the cells with β-caryophyllene in comparison with negative control and the standard reference drug, 5-flourouracil. The results were presented as mean ± SD, n = 3.
Figure 9
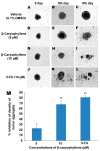
Anti-tumor aggregation effects of β-caryophyllene on in vitro HCT 116 cellular spheroids in a hanging drop assay. The cellular aggregates treated with vehicle (0.1% DMSO) developed in a solid spheroid shape within 9 days (A–C), whereas, β-caryophyllene at a concentration of 5 µM displayed significant inhibitory effect on the HCT 116 cellular aggregate microspheroids (D–F). At a concentration 10 µM, β-caryophyllene completely obliterated the solid cellular aggregates of HCT 116 cells on the 9th day of seeding (G–I). Similar effects were observed with the standard reference, 5-flourouracil (J–L). Graphical representation (M) of the dose dependent inhibitory effect of β-caryophyllene on in vitro cellular aggregates of HCT 116 cells on 9th day of treatment. The results are presented as mean ± SD, n = 6 (* p < 0.05, ** p < 0.01).
Similar articles
- Chemical composition and biological activity of the essential oil obtained from Bupleurum marginatum (Apiaceae).
Ashour ML, El-Readi M, Youns M, Mulyaningsih S, Sporer F, Efferth T, Wink M. Ashour ML, et al. J Pharm Pharmacol. 2009 Aug;61(8):1079-87. doi: 10.1211/jpp/61.08.0012. J Pharm Pharmacol. 2009. PMID: 19703352 - Identification and biological activity of the volatile compounds of Glycyrrhiza triphylla Fisch. & C.A.Mey.
Shakeri A, Akhtari J, Soheili V, Taghizadeh SF, Sahebkar A, Shaddel R, Asili J. Shakeri A, et al. Microb Pathog. 2017 Aug;109:39-44. doi: 10.1016/j.micpath.2017.05.022. Epub 2017 May 16. Microb Pathog. 2017. PMID: 28526637 - Chemical composition, antibacterial, antifungal and antioxidant activities of Algerian Eryngium tricuspidatum L. essential oil.
Merghache D, Boucherit-Otmani Z, Merghache S, Chikhi I, Selles C, Boucherit K. Merghache D, et al. Nat Prod Res. 2014;28(11):795-807. doi: 10.1080/14786419.2014.883392. Epub 2014 Feb 24. Nat Prod Res. 2014. PMID: 24559136 - β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties.
Fidyt K, Fiedorowicz A, Strządała L, Szumny A. Fidyt K, et al. Cancer Med. 2016 Oct;5(10):3007-3017. doi: 10.1002/cam4.816. Epub 2016 Sep 30. Cancer Med. 2016. PMID: 27696789 Free PMC article. Review. - One Hundred Faces of Geraniol.
Mączka W, Wińska K, Grabarczyk M. Mączka W, et al. Molecules. 2020 Jul 21;25(14):3303. doi: 10.3390/molecules25143303. Molecules. 2020. PMID: 32708169 Free PMC article. Review.
Cited by
- ZnO Nanocomposites of Juniperus procera and Dodonaea viscosa Extracts as Antiproliferative and Antimicrobial Agents.
Alghamdi MD, Nazreen S, Ali NM, Amna T. Alghamdi MD, et al. Nanomaterials (Basel). 2022 Feb 16;12(4):664. doi: 10.3390/nano12040664. Nanomaterials (Basel). 2022. PMID: 35214995 Free PMC article. - Cannabinoids as anticancer drugs: current status of preclinical research.
Hinz B, Ramer R. Hinz B, et al. Br J Cancer. 2022 Jul;127(1):1-13. doi: 10.1038/s41416-022-01727-4. Epub 2022 Mar 11. Br J Cancer. 2022. PMID: 35277658 Free PMC article. Review. - Antibacterial, Antioxidant Potency, and Chemical Composition of Essential Oils from Dried Powdered Leaves and Flowers of Hypericum revolutum subsp. keniense (Schweinf.).
Sengera GO, Kenanda EO, Onyancha JM. Sengera GO, et al. Evid Based Complement Alternat Med. 2023 Jan 3;2023:4125885. doi: 10.1155/2023/4125885. eCollection 2023. Evid Based Complement Alternat Med. 2023. PMID: 36636606 Free PMC article. - Phytochemical Compositions and Antioxidant Activities of Essential Oils Extracted from the Flowers of Paeonia delavayi Using Supercritical Carbon Dioxide Fluid.
Yu X, Zhang H, Wang J, Wang J, Wang Z, Li J. Yu X, et al. Molecules. 2022 May 7;27(9):3000. doi: 10.3390/molecules27093000. Molecules. 2022. PMID: 35566350 Free PMC article. - Agarwood Essential Oil Ameliorates Restrain Stress-Induced Anxiety and Depression by Inhibiting HPA Axis Hyperactivity.
Wang S, Wang C, Yu Z, Wu C, Peng D, Liu X, Liu Y, Yang Y, Guo P, Wei J. Wang S, et al. Int J Mol Sci. 2018 Nov 5;19(11):3468. doi: 10.3390/ijms19113468. Int J Mol Sci. 2018. PMID: 30400578 Free PMC article.
References
- Chen H., Yang Y., Xue J., Wei J., Zhang Z., Chen H. Comparison of compositions and antimicrobial activities of essential oils from chemically stimulated agarwood, wild agarwood and healthy Aquilaria sinensis (Lour.) Gilg trees. Molecules. 2011;16:4884–4896. doi: 10.3390/molecules16064884. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous