Berberine inhibits hepatic gluconeogenesis via the LKB1-AMPK-TORC2 signaling pathway in streptozotocin-induced diabetic rats - PubMed (original) (raw)
Berberine inhibits hepatic gluconeogenesis via the LKB1-AMPK-TORC2 signaling pathway in streptozotocin-induced diabetic rats
Shu-Jun Jiang et al. World J Gastroenterol. 2015.
Abstract
Aim: To investigate the molecular mechanisms of berberine inhibition of hepatic gluconeogenesis in a diabetic rat model.
Methods: The 40 rats were randomly divided into five groups. One group was selected as the normal group. In the remaining groups (n = 8 each), the rats were fed on a high-fat diet for 1 mo and received intravenous injection of streptozotocin for induction of the diabetic models. Berberine (156 mg/kg per day) (berberine group) or metformin (184 mg/kg per day) (metformin group) was intragastrically administered to the diabetic rats and 5-aminoimidazole-4-carboxamide1-β-D-ribofuranoside (AICAR) (0.5 mg/kg per day) (AICAR group) was subcutaneously injected to the diabetic rats for 12 wk. The remaining eight diabetic rats served as the model group. Fasting plasma glucose and insulin levels as well as lipid profile were tested. The expressions of proteins were examined by western blotting. The nuclear translocation of CREB-regulated transcription co-activator (TORC)2 was observed by immunohistochemical staining.
Results: Berberine improved impaired glucose tolerance and decreased plasma hyperlipidemia. Moreover, berberine decreased fasting plasma insulin and homeostasis model assessment of insulin resistance (HOMA-IR). Berberine upregulated protein expression of liver kinase (LK)B1, AMP-activated protein kinase (AMPK) and phosphorylated AMPK (p-AMPK). The level of phophorylated TORC2 (p-TORC2) protein in the cytoplasm was higher in the berberine group than in the model group, and no significant difference in total TORC2 protein level was observed. Immunohistochemical staining revealed that more TORC2 was localized in the cytoplasm of the berberine group than in the model group. Moreover, berberine treatment downregulated protein expression of the key gluconeogenic enzymes (phosphoenolpyruvate carboxykinase and glucose-6-phosphatase) in the liver tissues.
Conclusion: Our findings revealed that berberine inhibited hepatic gluconeogenesis via the regulation of the LKB1-AMPK-TORC2 signaling pathway.
Keywords: AMPK; Berberine; Diabetes; Hepatic gluconeogenesis; LKB1; TORC2.
Figures
Figure 1
Effects of berberine on plasma glucose levels in the oral glucose tolerance test and the areas under the curves for plasma glucose. b_P_ < 0.01 vs the normal control group at the corresponding time point; d_P_ < 0.01 vs the model group at the corresponding time point (by ANOVA). AUC: Areas under the curve.
Figure 2
Effects of berberine on fasting plasma insulin level and homeostasis model assessment of insulin resistance in diabetic rats. Each bar represents the mean ± SD (n = 8). b_P_ < 0.01 vs the normal control group; d_P_ < 0.01 vs the model group (by ANOVA). HOMA-IR: Homeostasis model assessment of insulin resistance.
Figure 3
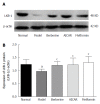
Effect of berberine on hepatic LKB-1 protein expression. Western blot analyses of LKB-1 levels in liver tissues of normal control rats, model rats and diabetic rats treated with berberine, AICAR and metformin. A: Representative blots for each group are shown; B: Each bar is expressed as LKB-1/β-actin and represents the mean ± SD (n = 8). a_P_ < 0.05 vs the normal control group; c_P_ < 0.05 vs the model group (by ANOVA).
Figure 4
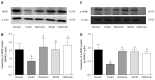
Effect of berberine on hepatic AMPK and p-AMPK protein expression. Western blot analyses of AMPK and p-AMPK protein in liver tissues of normal control rats, model rats and diabetic rats treated with berberine, AICAR or metformin. A, C: Representative blots for each group are shown; B: Each bar is expressed as AMPKD/β-actin and represents the mean ± SD (n = 8); D: Each bar is expressed as p-AMPK/β-actin and represents the mean ± SD (n = 8). b_P_ < 0.01 vs the normal control group; d_P_ < 0.01 vs the model group (by ANOVA).
Figure 5
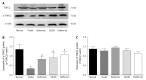
Effect of berberine on hepatic p-TORC2 and total TORC2 protein expression. Western blot analyses of p-TORC2 and TORC2 proteins from liver tissues of normal rats, model rats and diabetic rats treated with Berberine, AICAR and Metformin. A: Representative blots for each group are shown; B: Each bar is expressed as p-TORC2/β-actin and represents the mean ± SD (n = 8); C: Each bar is expressed as total TORC2/β-actin and represents the mean ± SD (n = 8). b_P_ < 0.01 vs the normal group; d_P_ < 0.01 vs the model group (by ANOVA).There was no significant difference in the expression of total TORC2 protein across the five groups.
Figure 6
Immunohistochemical staining for TORC2 in the liver tissues. Optical microscopy image of TORC2 is shown in brown. The normal group (A) exhibited little TORC2 in the nuclei. However, more TORC2 was present in the nuclei of the model group (B). The groups treated with Berberine (C), AICAR (D) and Metformin (E) exhibited lower levels of TORC2 compared to the model group (magnification × 400).
Figure 7
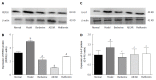
Berberine inhibited expression of key gluconeogenic enzyme proteins. Western blot analyses of PEPCK and G-6-P proteins in liver tissues of normal rats, model rats and diabetic rats treated with berberine, AICAR or metformin. A: PEPCK blots for each group are shown; C: G-6-P blots for each group are shown; B: Each bar is expressed as the total PEPCK/β-actin and represents the mean ± SD (n = 8); D: Each bar is expressed as the total G-6-P/β-actin and represents the mean ± SD (n = 8). b_P_ < 0.01 vs normal control group; d_P_ < 0.01 vs model group; a_P_ < 0.05 vs normal control group; c_P_ < 0.05 vs model group (by ANOVA).
Similar articles
- Berberine Inhibits Gluconeogenesis in Skeletal Muscles and Adipose Tissues in Streptozotocin-induced Diabetic Rats via LKB1-AMPK-TORC2 Signaling Pathway.
Xu XH, Hu Q, Zhou LS, Xu LJ, Zou X, Lu FE, Yi P. Xu XH, et al. Curr Med Sci. 2020 Jun;40(3):530-538. doi: 10.1007/s11596-020-2210-4. Epub 2020 Jul 17. Curr Med Sci. 2020. PMID: 32681256 - Sodium caprate augments the hypoglycemic effect of berberine via AMPK in inhibiting hepatic gluconeogenesis.
Zhang M, Lv X, Li J, Meng Z, Wang Q, Chang W, Li W, Chen L, Liu Y. Zhang M, et al. Mol Cell Endocrinol. 2012 Nov 5;363(1-2):122-30. doi: 10.1016/j.mce.2012.08.006. Epub 2012 Aug 16. Mol Cell Endocrinol. 2012. PMID: 22922125 Free PMC article. - Combretum lanceolatum flowers ethanol extract inhibits hepatic gluconeogenesis: an in vivo mechanism study.
Siqueira JT, Batistela E, Pereira MP, da Silva VC, de Sousa Junior PT, Andrade CM, Kawashita NH, Bertolini GL, Baviera AM. Siqueira JT, et al. Pharm Biol. 2016 Sep;54(9):1671-9. doi: 10.3109/13880209.2015.1120321. Epub 2016 Feb 10. Pharm Biol. 2016. PMID: 26864726 - New Insight into the Effects of Metformin on Diabetic Retinopathy, Aging and Cancer: Nonapoptotic Cell Death, Immunosuppression, and Effects beyond the AMPK Pathway.
Hsu SK, Cheng KC, Mgbeahuruike MO, Lin YH, Wu CY, Wang HD, Yen CH, Chiu CC, Sheu SJ. Hsu SK, et al. Int J Mol Sci. 2021 Aug 31;22(17):9453. doi: 10.3390/ijms22179453. Int J Mol Sci. 2021. PMID: 34502359 Free PMC article. Review. - AMPK inhibits liver gluconeogenesis: fact or fiction?
Johanns M, Hue L, Rider MH. Johanns M, et al. Biochem J. 2023 Jan 13;480(1):105-125. doi: 10.1042/BCJ20220582. Biochem J. 2023. PMID: 36637190 Review.
Cited by
- Role of glycolysis in diabetic atherosclerosis.
Liu QJ, Yuan W, Yang P, Shao C. Liu QJ, et al. World J Diabetes. 2023 Oct 15;14(10):1478-1492. doi: 10.4239/wjd.v14.i10.1478. World J Diabetes. 2023. PMID: 37970130 Free PMC article. Review. - Protective effects of asiatic acid in a spontaneous type 2 diabetic mouse model.
Sun W, Xu G, Guo X, Luo G, Wu L, Hou Y, Guo X, Zhou J, Xu T, Qin L, Fan Y, Han L, Matsabisa M, Ma X, Liu T. Sun W, et al. Mol Med Rep. 2017 Aug;16(2):1333-1339. doi: 10.3892/mmr.2017.6684. Epub 2017 Jun 2. Mol Med Rep. 2017. PMID: 28586016 Free PMC article. - An Overview of Herbal-Based Antidiabetic Drug Delivery Systems: Focus on Lipid- and Inorganic-Based Nanoformulations.
Kambale EK, Quetin-Leclercq J, Memvanga PB, Beloqui A. Kambale EK, et al. Pharmaceutics. 2022 Oct 8;14(10):2135. doi: 10.3390/pharmaceutics14102135. Pharmaceutics. 2022. PMID: 36297570 Free PMC article. Review. - Rhizoma coptidis as a Potential Treatment Agent for Type 2 Diabetes Mellitus and the Underlying Mechanisms: A Review.
Ran Q, Wang J, Wang L, Zeng HR, Yang XB, Huang QW. Ran Q, et al. Front Pharmacol. 2019 Jul 22;10:805. doi: 10.3389/fphar.2019.00805. eCollection 2019. Front Pharmacol. 2019. PMID: 31396083 Free PMC article. Review. - Cellular stress response mechanisms of Rhizoma coptidis: a systematic review.
Wang J, Ran Q, Zeng HR, Wang L, Hu CJ, Huang QW. Wang J, et al. Chin Med. 2018 Jun 7;13:27. doi: 10.1186/s13020-018-0184-y. eCollection 2018. Chin Med. 2018. PMID: 29930696 Free PMC article. Review.
References
- Radziuk J, Pye S. Hepatic glucose uptake, gluconeogenesis and the regulation of glycogen synthesis. Diabetes Metab Res Rev. 2001;17:250–272. - PubMed
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. - PubMed
- Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M, Höglund P, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–187. - PubMed
- Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases